Uses
Temporary relief of occasional headaches and minor aches and pain with accompanying sleeplessness
Warnings
Alcohol Warnings
If you consume 3 or more alcoholic drinks every day, ask your doctor if you should take acetaminophen or other pain relievers/fever reducers. Acetaminophen may cause liver damage.
Do not use
- *
- with any other product containing acetaminophen
- *
- with any other product containing diphenhydramine, even one used on skin
- *
- in children under 12 years of age
Ask a doctor before use if you have
- *
- a breathing problem such as emphysema or chronic bronchitis
- *
- glaucoma
- *
- difficulty in urination due to enlargement of the prostate gland
When using this product
- *
- do not exceed recommended dosage
- *
- avoid alcoholic beverages
- *
- marked drowsiness may occur
- *
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- *
- sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of a serious underlying medical illness
- *
- new symptoms occur
- *
- redness or swelling is present
- *
- pain gets worse or lasts more than 10 days
- *
- fever gets worse or lasts more than 3 days
Direction
- *
- use as directed
- *
- adults and children 12 years and over : take 2 caplets at bedtime or as directed by a doctor
- *
- Children under 12 years : do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and could cause serious health problems.
Inactive ingredients
croscarmellose sodium, hypromellose, polythlene glycol,sodium metabisulfate, stearic acid,, sodium starch glycolate,collodial silicon dioxide, FD & C blue # 1
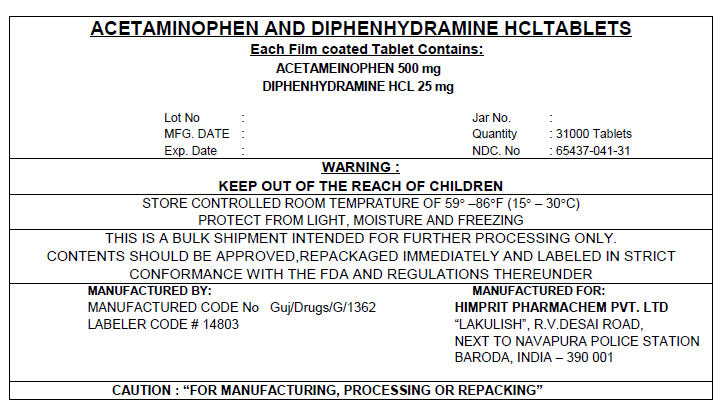
PRINCIPAL DISPLAY PANEL 500/25 mg Shipper Label
ACETAMINOPHEN AND DIPHENHYDRAMINE HCL TABLETS
Each Film coated Tablet Contains:
ACETAMEINOPHEN 500 mg
DIPHENHYDRAMINE HCL 25 mg
Lot No :
MFG. DATE :
Exp. Date :
Jar No. :
Quantity : 31000 Tablets
NDC. No : 65437-041-31
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° –86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FDA AND REGULATIONS THEREUNDER
MANUFACTURED BY:
MANUFACTURED CODE No Guj/Drugs/G/1362
LABELER CODE # 14803
MANUFACTURED FOR:
HIMPRIT PHARMACHEM PVT. LTD
"LAKULISH", R.V.DESAI ROAD,
NEXT TO NAVAPURA POLICE STATION
BARODA, INDIA – 390 001
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"