Label: ALORA SENSITIVITY- potassium nitrate and sodium fluoride gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 72288-309-01, 72288-309-02, 72288-309-03 - Packager: Amazon, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- Adults and children 12 years of age and older: Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth.
- Children under 12 years of age: Consult a dentist or doctor.
- Other Information
- Inactive Ingredients
- Questions or comments
- SPL UNCLASSIFIED SECTION
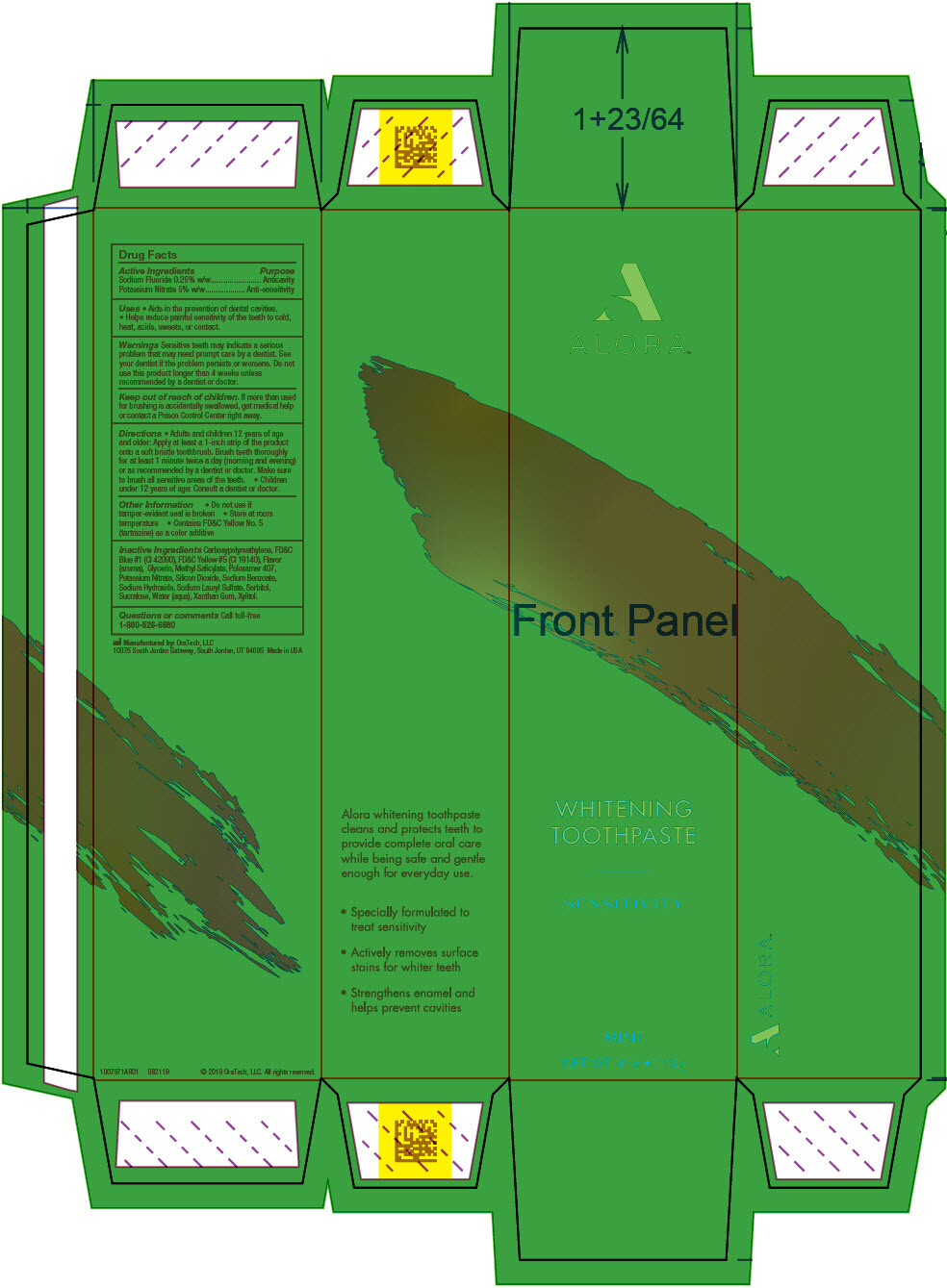
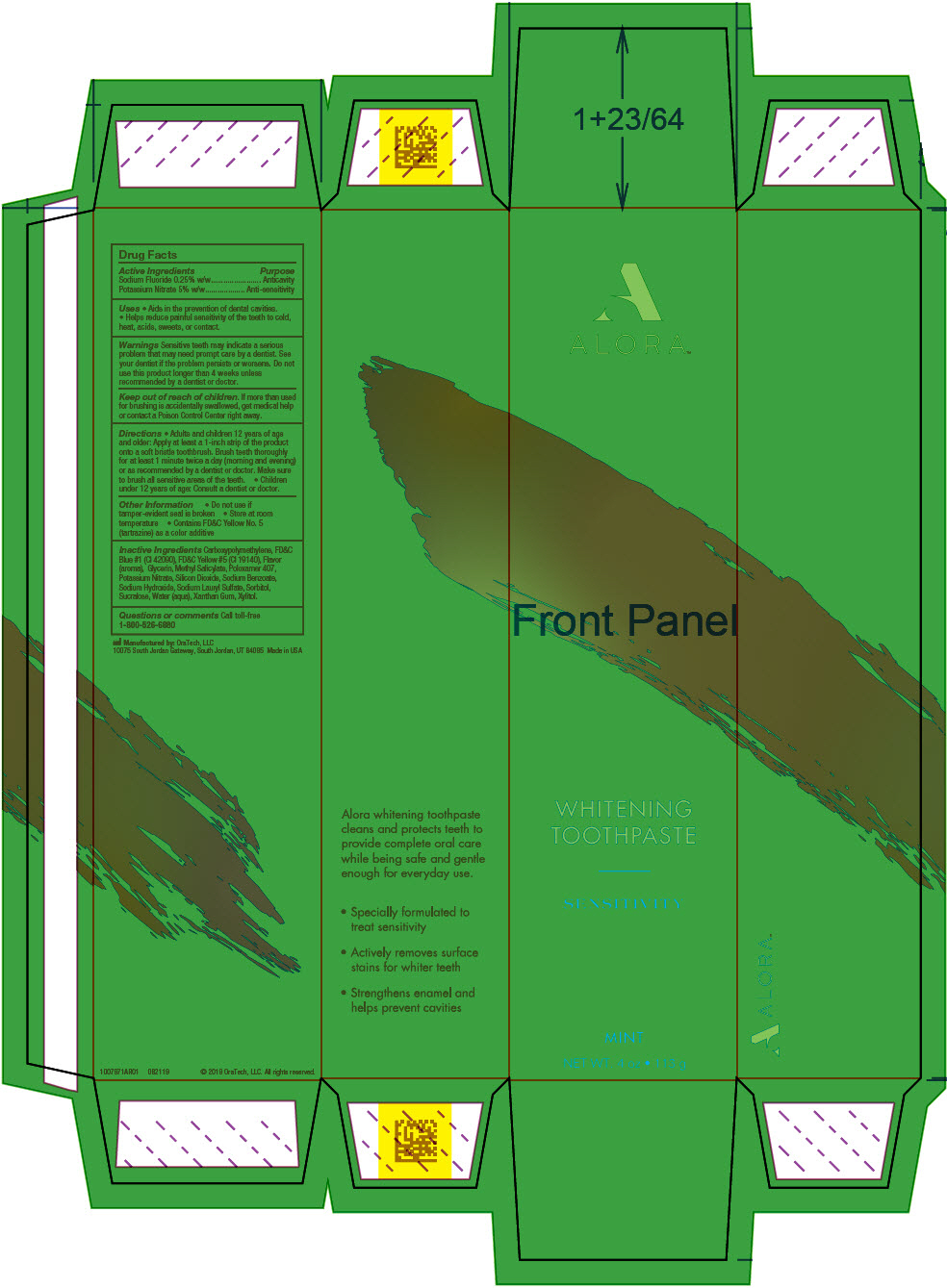
- PRINCIPAL DISPLAY PANEL - 113 g Tube Carton
-
INGREDIENTS AND APPEARANCE
ALORA SENSITIVITY
potassium nitrate and sodium fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72288-309 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium Nitrate (UNII: RU45X2JN0Z) (Nitrate Ion - UNII:T93E9Y2844) Potassium Nitrate 50 mg in 1 g Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2.5 mg in 1 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) Water (UNII: 059QF0KO0R) Silicon Dioxide (UNII: ETJ7Z6XBU4) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) Poloxamer 407 (UNII: TUF2IVW3M2) Methyl Salicylate (UNII: LAV5U5022Y) Sodium Lauryl Sulfate (UNII: 368GB5141J) Xanthan Gum (UNII: TTV12P4NEE) Sodium Benzoate (UNII: OJ245FE5EU) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) Sodium Hydroxide (UNII: 55X04QC32I) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color GREEN Score Shape Size Flavor MINT (Cool Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-309-02 3 in 1 PACKAGE, COMBINATION 10/01/2019 1 NDC:72288-309-01 1 in 1 CARTON 1 28.35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:72288-309-03 6 in 1 PACKAGE, COMBINATION 10/01/2019 2 NDC:72288-309-01 1 in 1 CARTON 2 28.35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 10/01/2019 Labeler - Amazon, Inc (128990418)