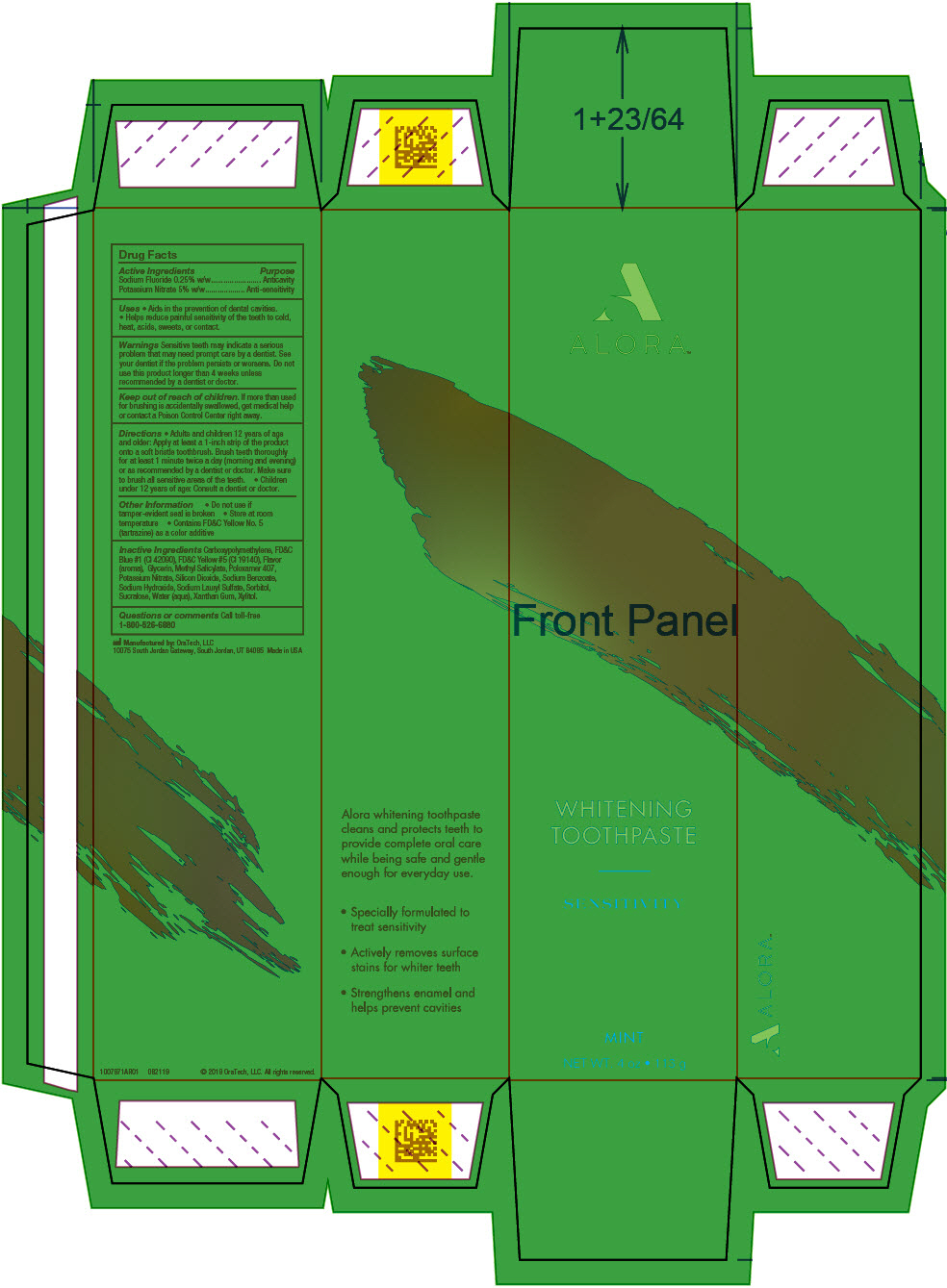
Uses
- Aids in the prevention of dental cavities.
- Helps reduce painful sensitivity of the teeth to cold, heat, acids, sweets, or contact.
Warnings
Sensitive teeth may indicate a serious problem that may need prompt care by a dentist. See your dentist if the problem persists or worsens. Do not use this product longer than 4 weeks unless recommended by a dentist or doctor.
Directions
- Adults and children 12 years of age and older: Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth.
- Children under 12 years of age: Consult a dentist or doctor.
Other Information
- Do not use if tamper-evident seal is broken
- Store at room temperature
- Contains FD&C Yellow No. 5 (tartrazine) as a color additive
Inactive Ingredients
Carboxypolymethylene, FD&C Blue #1 (CI 42090), FD&C Yellow #5 (CI 19140), Flavor (aroma), Glycerin, Methyl Salicylate, Poloxamer 407, Potassium Nitrate, Silicon Dioxide, Sodium Benzoate, Sodium Hydroxide, Sodium Lauryl Sulfate, Sorbitol, Sucralose, Water (aqua), Xanthan Gum, Xylitol.