Label: ALEVE BACK AND MUSCLE PAIN- naproxen sodium tablet
-
NDC Code(s):
0280-6092-02,
0280-6092-04,
0280-6092-05,
0280-6092-06, view more0280-6092-07, 0280-6092-08, 0280-6092-09, 0280-6092-10, 0280-6092-12, 0280-6092-24, 0280-6092-25, 0280-6092-50
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
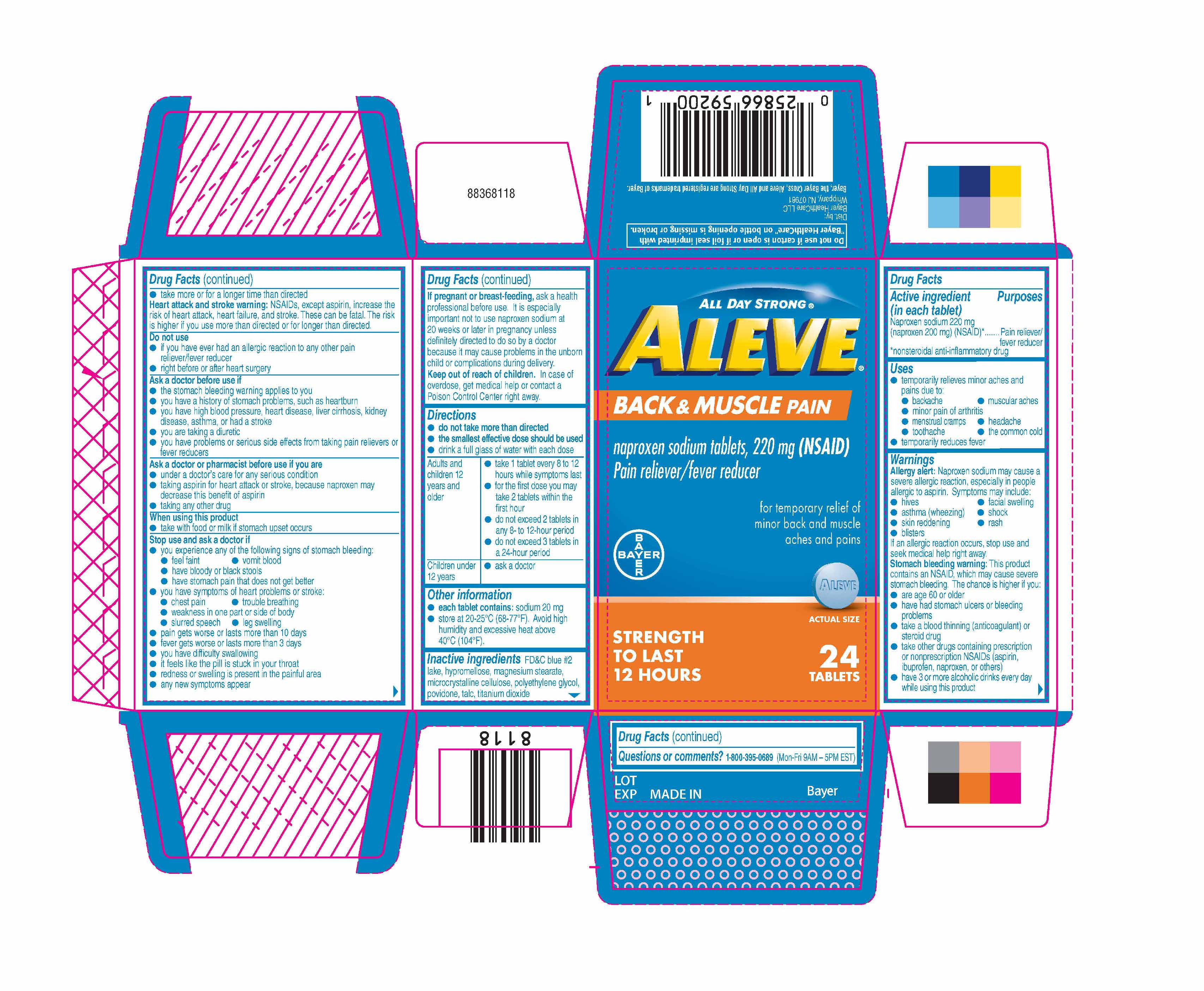
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
DOSAGE & ADMINISTRATION
Directions
- do not take more than directed
- the smallest effective dose should be used
- drink a full glass of water with each dose
Adults and children 12 years and older - take 1 tablets every 8 to 12 hours while symptoms last
- for the first dose you may take 2 tablets within the first hour
- do not exceed 2 tablets in any 8-to 12-hour period
- do not exceed 3 tablets in a 24-hour period
Children under 12 years - ask a doctor
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Package label display
-
INGREDIENTS AND APPEARANCE
ALEVE BACK AND MUSCLE PAIN
naproxen sodium tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-6092 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN SODIUM (UNII: 9TN87S3A3C) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN SODIUM 220 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POVIDONE (UNII: FZ989GH94E) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color blue Score no score Shape ROUND Size 8mm Flavor Imprint Code ALEVE Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-6092-24 1 in 1 CARTON 01/26/2018 1 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:0280-6092-50 1 in 1 CARTON 01/26/2018 2 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:0280-6092-10 1 in 1 CARTON 01/26/2018 09/30/2023 3 100 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:0280-6092-25 1 in 1 CARTON 08/06/2019 4 250 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:0280-6092-02 1 in 1 CARTON 01/26/2018 5 130 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:0280-6092-04 1 in 1 CARTON 01/26/2018 6 200 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:0280-6092-05 1 in 1 CARTON 01/26/2018 7 225 in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:0280-6092-06 1 in 1 CARTON 01/26/2018 03/31/2022 8 36 in 1 BOTTLE; Type 0: Not a Combination Product 9 NDC:0280-6092-07 1 in 1 CARTON 01/26/2018 11/30/2021 9 65 in 1 BOTTLE; Type 0: Not a Combination Product 10 NDC:0280-6092-08 1 in 1 CARTON 07/01/2021 10 250 in 1 BOTTLE; Type 0: Not a Combination Product 11 NDC:0280-6092-09 1 in 1 CARTON 07/01/2021 11 90 in 1 BOTTLE; Type 0: Not a Combination Product 12 NDC:0280-6092-12 10 in 1 VIAL; Type 0: Not a Combination Product 07/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020204 01/26/2018 Labeler - Bayer HealthCare LLC. (112117283)

 All Day Strong®
All Day Strong®