Active ingredient (in each tablet)
Naproxen sodium 220 mg (naproxen 200 mg)
(NSAID)*
*nonsteroidal anti-inflammatory drug
Purposes
Pain reliever/fever reducer
Uses
- temporarily relieves minor aches and pains due to:
- backache
- muscular aches
- minor pain of arthritis
- menstrual cramps
- headache
- toothache
- the common cold
- temporarily reduces fever
Directions
-
do not take more than directed
-
the smallest effective dose should be used
- drink a full glass of water with each dose
| Adults and children 12 years and older |
- take 1 tablets every 8 to 12 hours while symptoms last
- for the first dose you may take 2 tablets within the first hour
- do not exceed 2 tablets in any 8-to 12-hour period
- do not exceed 3 tablets in a 24-hour period
|
| Children under 12 years |
|
Other information
-
each tablet contains: sodium 20 mg
- store at 20-25ºC (68-77ºF). Avoid high humidity and excessive heat above 40°C (104°F).
Inactive ingredients FD&C blue #2 lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, talc, titanium dioxide
Questions or comments? 1-800-395-0689 (Mon – Fri 9AM – 5PM EST)
Dist. by :
Bayer Healthcare LLC
Whippany, NJ 07981
In case of overdose, get medical help or contact a Poison Control Center right away.
Package label display
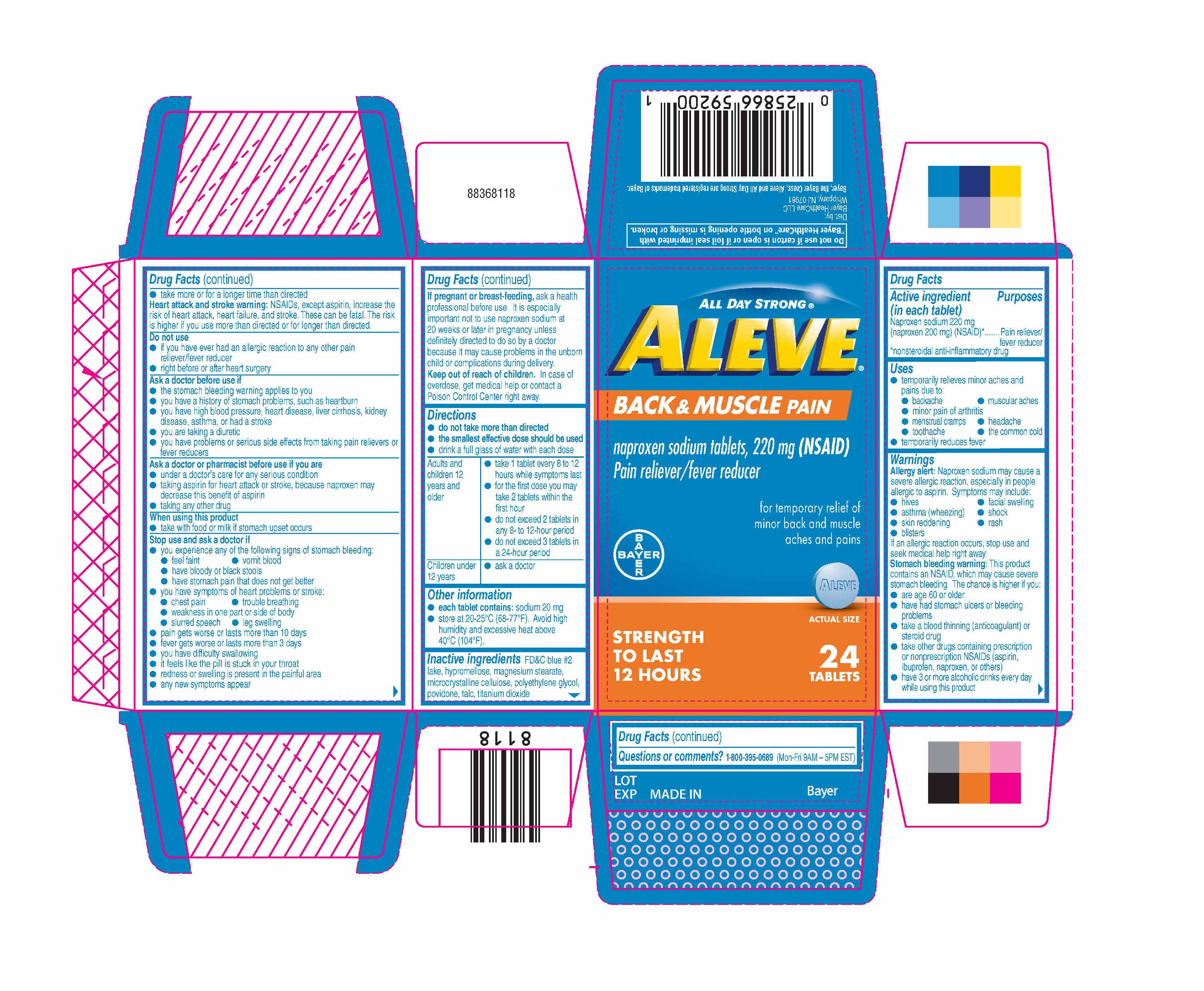 All Day Strong®
All Day Strong®
Aleve®
Back & Muscle Pain
naproxen sodium tablets, 220 mg (NSAID)
Pain reliever/fever reducer
Bayer
For temporary relief of minor back and muschle aches and pains
ACTUAL SIZE
Strength to Last 12 Hours 24Tablets
Bayer HealthCare LLC.
 All Day Strong®
All Day Strong®