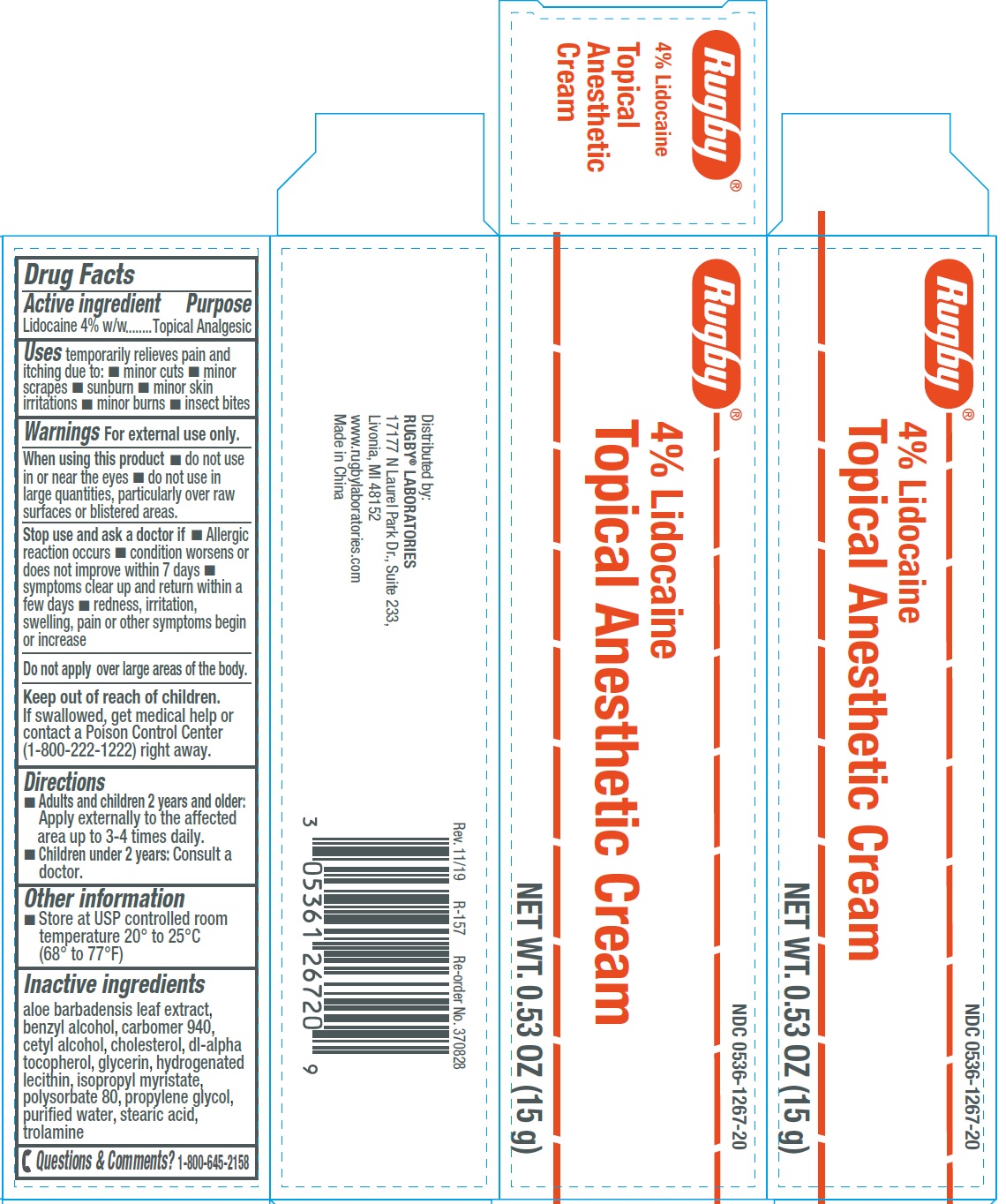
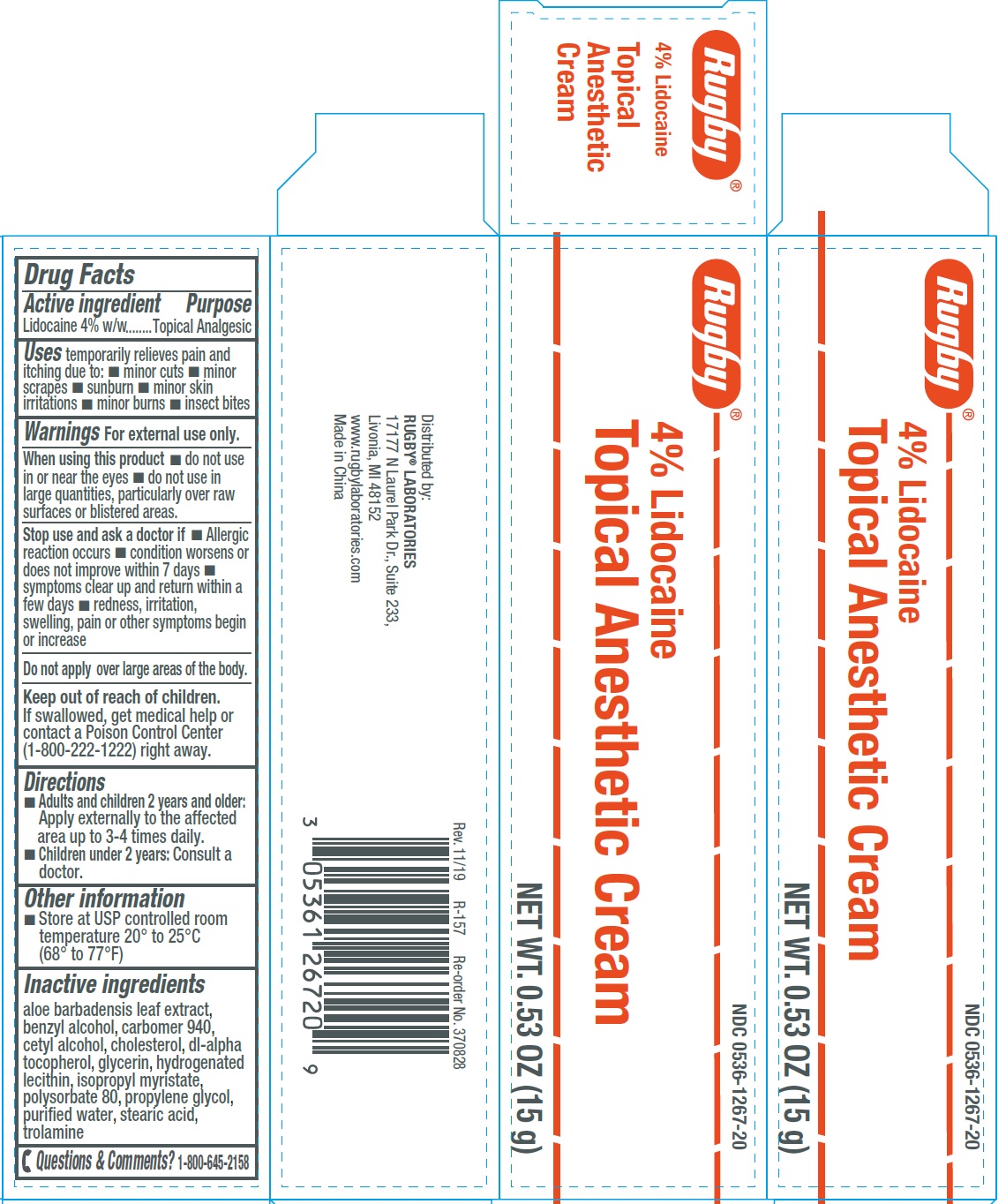
Label: 4 LIDOCAINE TOPICAL ANESTHETIC- lidocaine cream
- NDC Code(s): 0536-1267-20
- Packager: RUGBY LABORATORIES
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas.
- Directions
- Other information
- Inactive ingredients
- Questions & Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
4 LIDOCAINE TOPICAL ANESTHETIC
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1267 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CETYL ALCOHOL (UNII: 936JST6JCN) CHOLESTEROL (UNII: 97C5T2UQ7J) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1267-20 15 g in 1 BOX; Type 0: Not a Combination Product 04/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/30/2020 Labeler - RUGBY LABORATORIES (079246066)