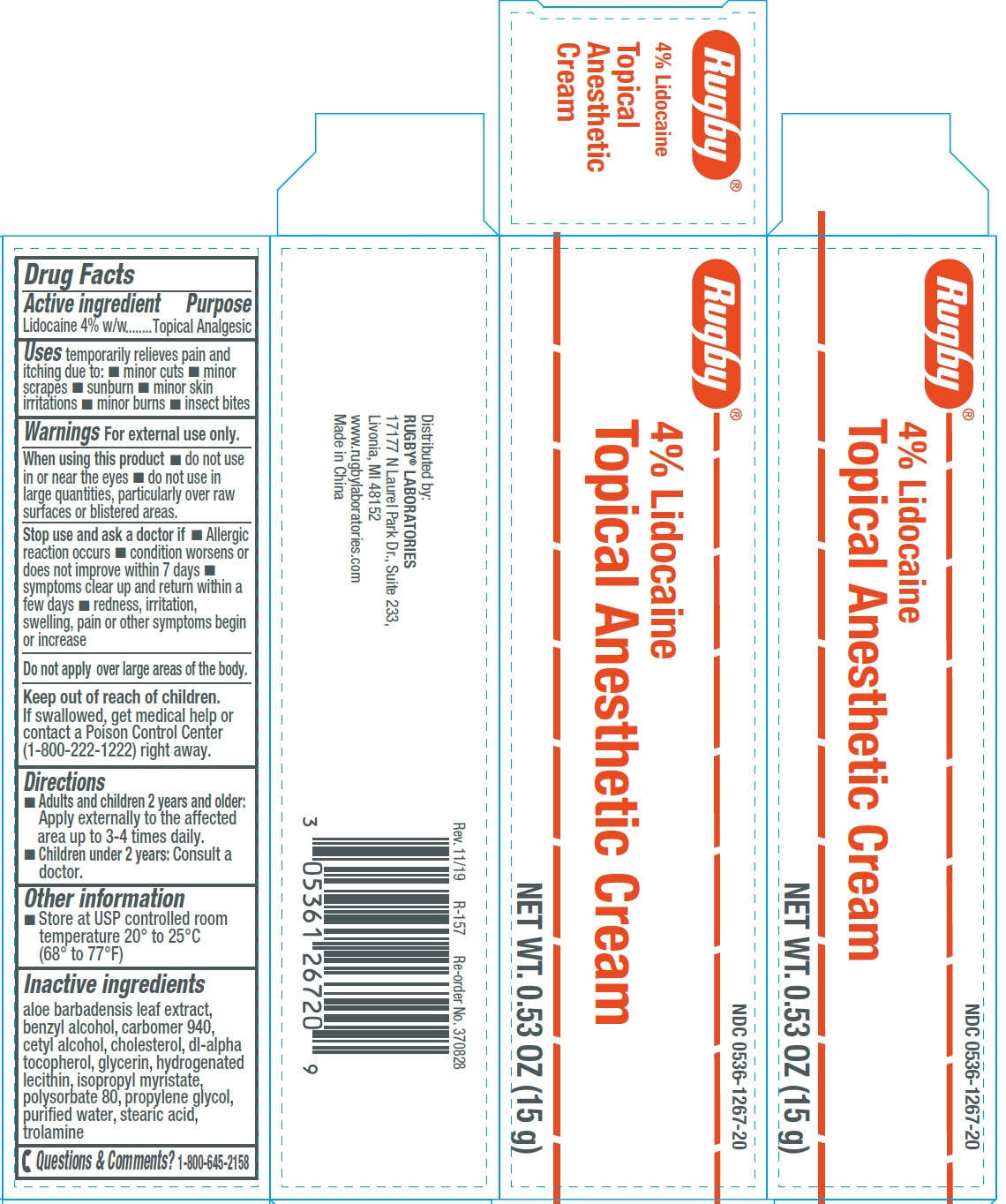
Active ingredient
Active ingredient
Purpose
Topical Analgesic
Uses
temporarily relieves pain and itching due to:
- minor cuts
- minor scrapes
- sunburn
- minor skin irritations
- minor burns
- insect bites
Warnings
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas.
Stop use and ask a doctor if
- Allergic reaction occurs
- condition worsens or does not improve within 7 days
- symptoms clear up and return within a few days
- redness, irritation, swelling, pain or other symptoms begin or increase
Do not apply over large areas of the body.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- Adults and children 2 years and older: Apply externally to the affected area up to 3-4 times daily.
- Children under 2 years: Consult a doctor.
Other information
- Store at USP controlled room temperature 20° to 25°C (68° to 77°F)
Inactive ingredients
aloe barbadensis leaf extract, benzyl alcohol, carbomer 940, cetyl alcohol, cholesterol, di-alpha tocopherol, glycerin, hydrogenated lecithin, isopropyl myristate, polysorbate 80, propylene glycol, purified water, stearic acid, trolamine
Questions & Comments?
1-800-645-2158
Package Labeling: