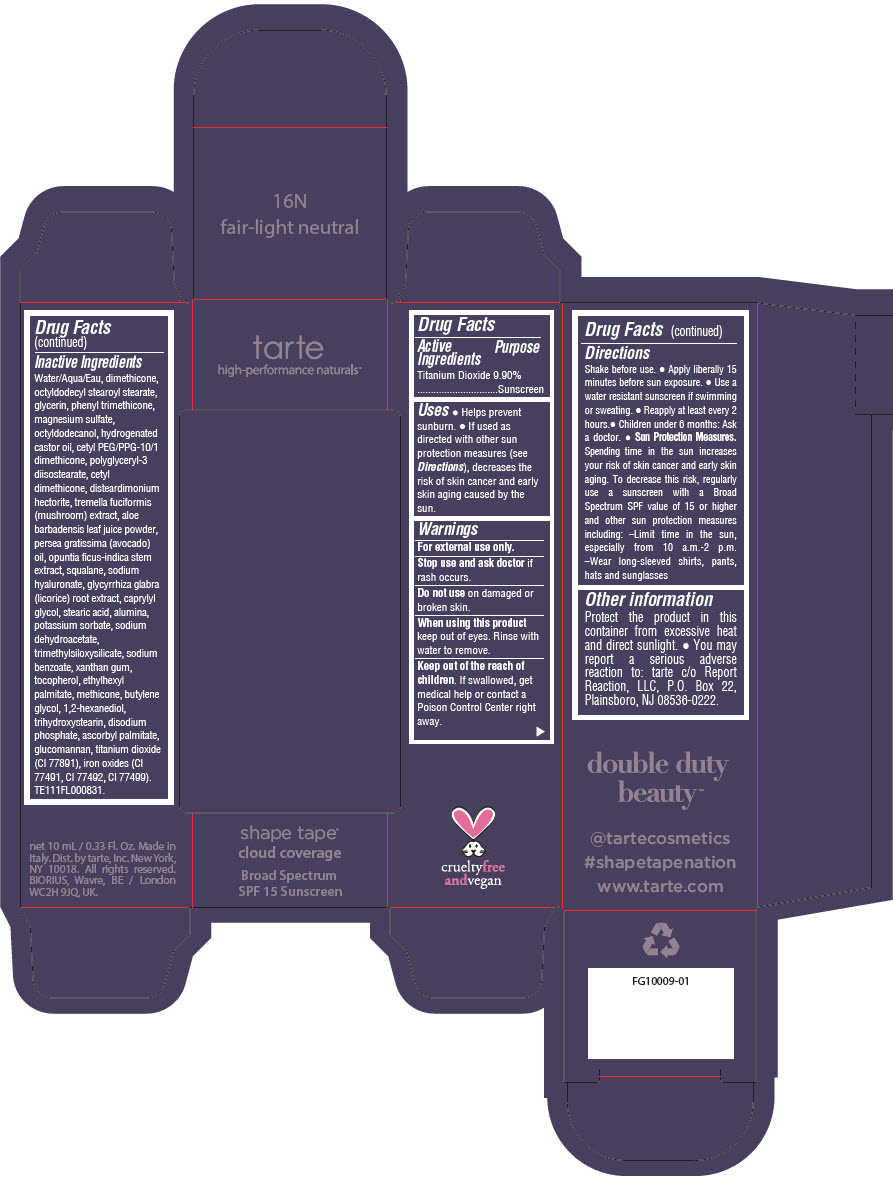
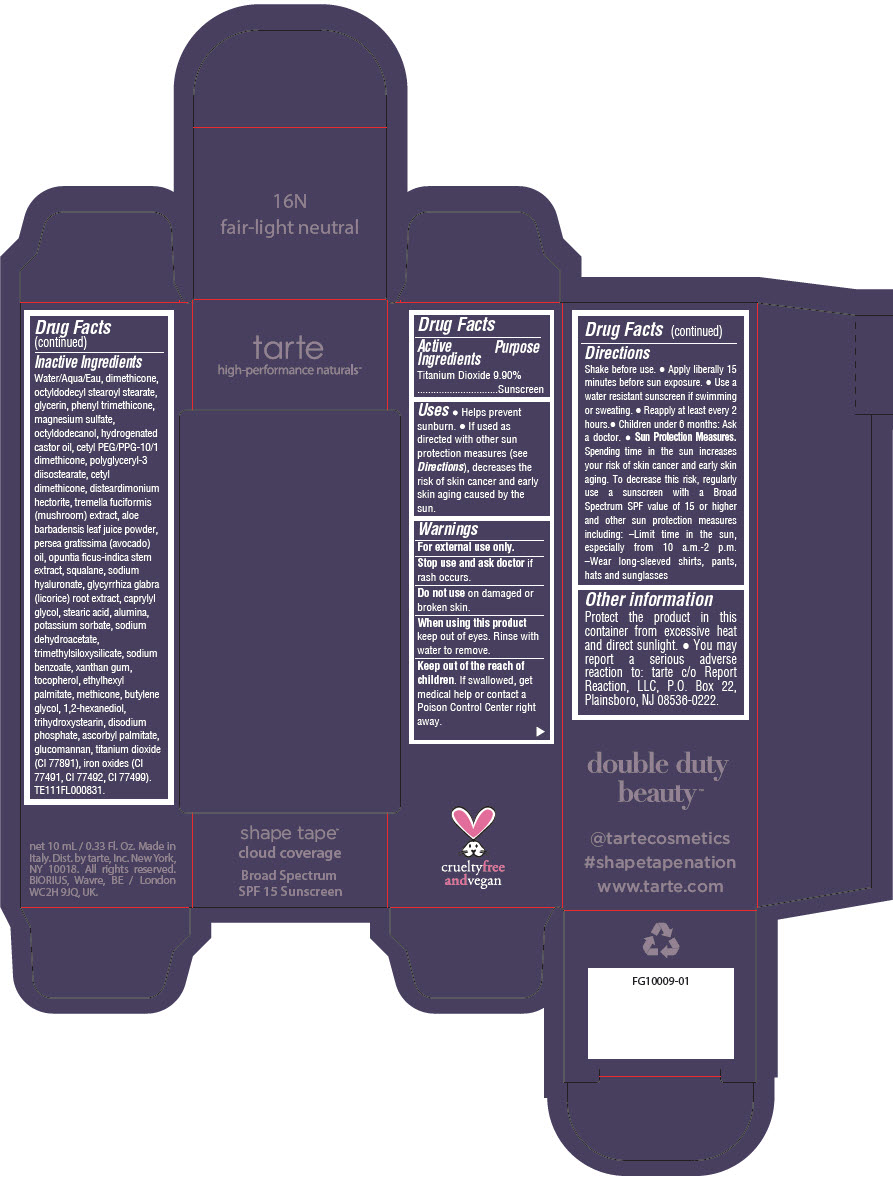
Label: DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 16N FAIR-LIGHT NEUTRAL- titanium dioxide liquid
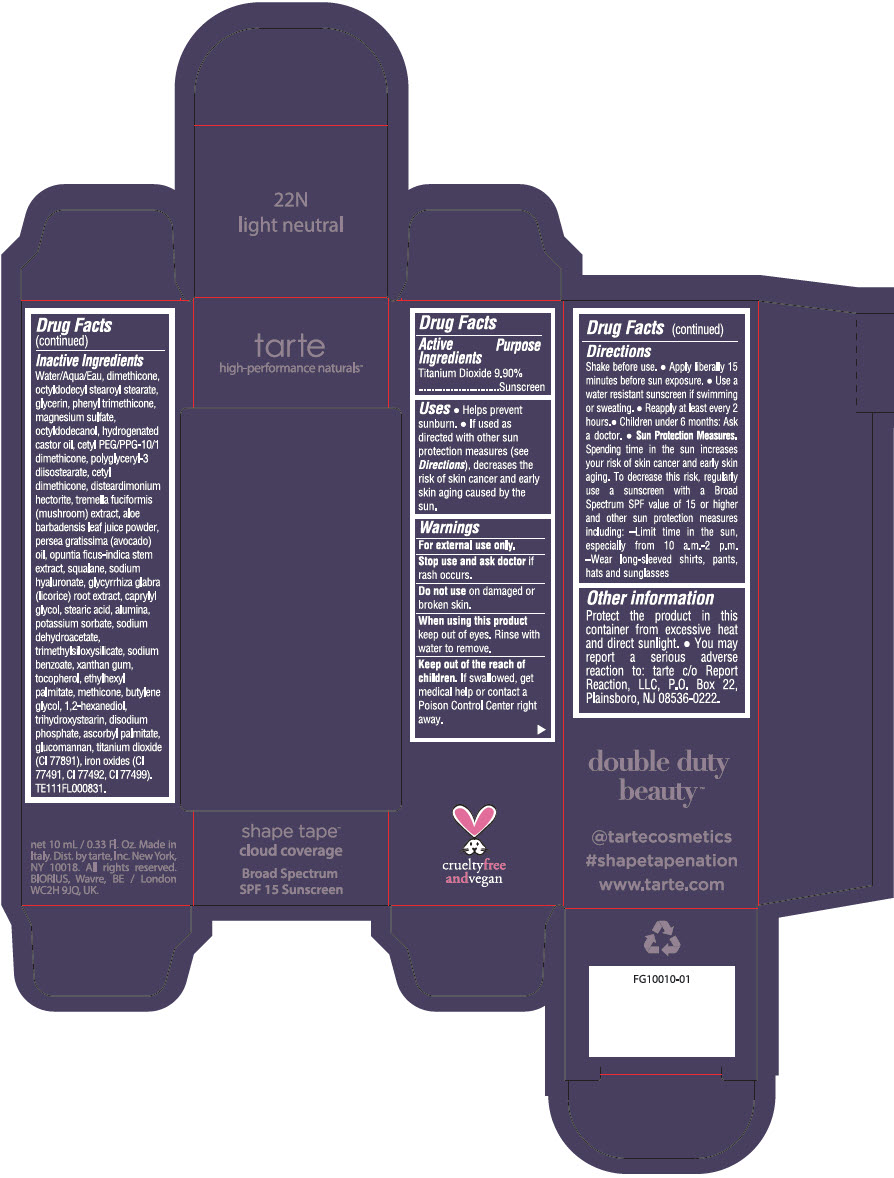
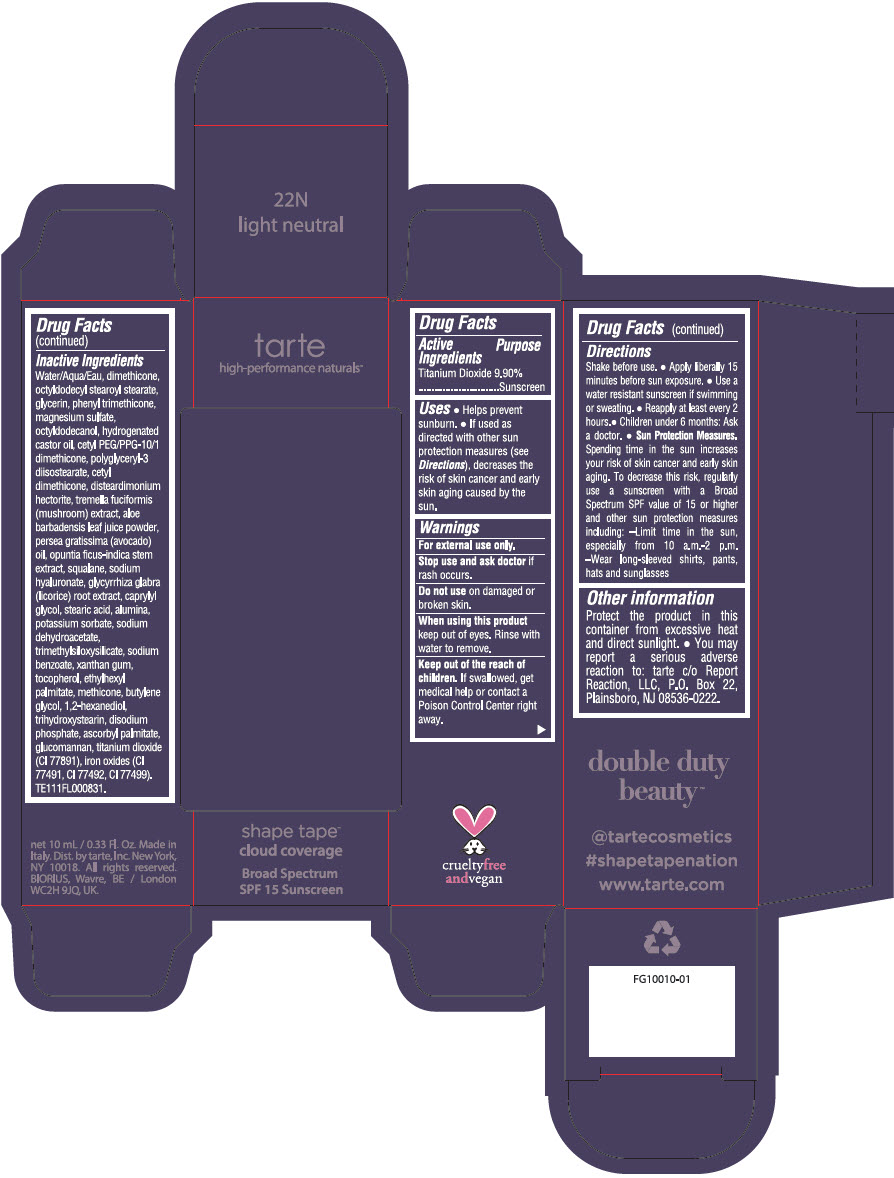
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 22N LIGHT NEUTRAL- titanium dioxide liquid
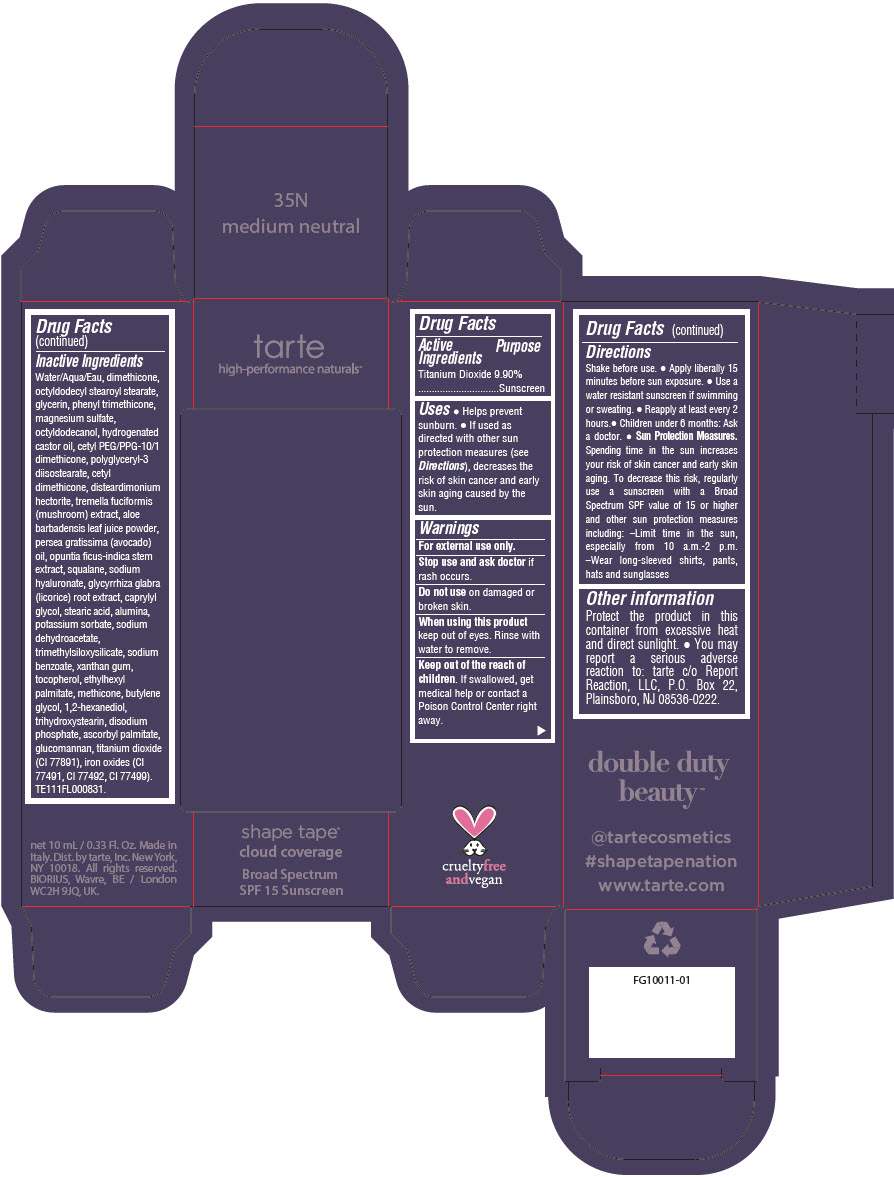
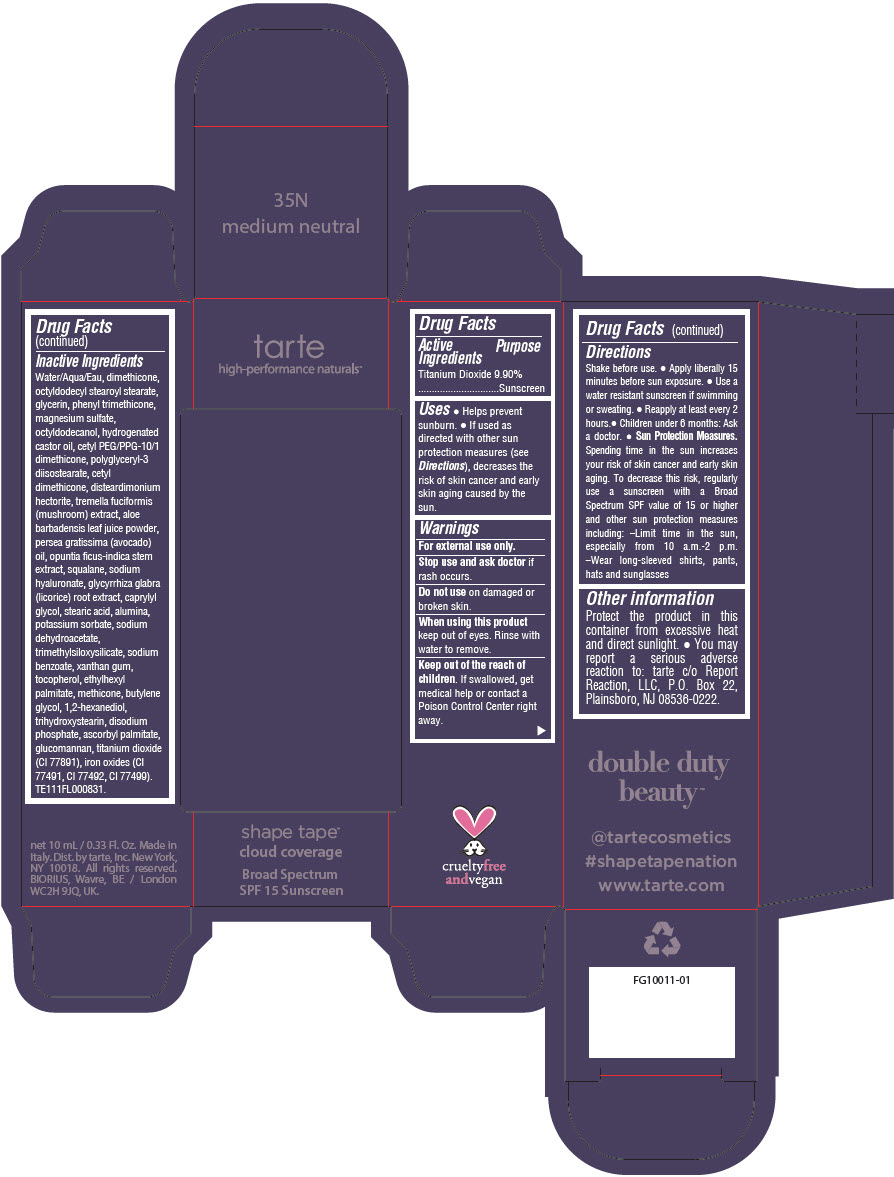
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 35N MEDIUM NEUTRAL- titanium dioxide liquid
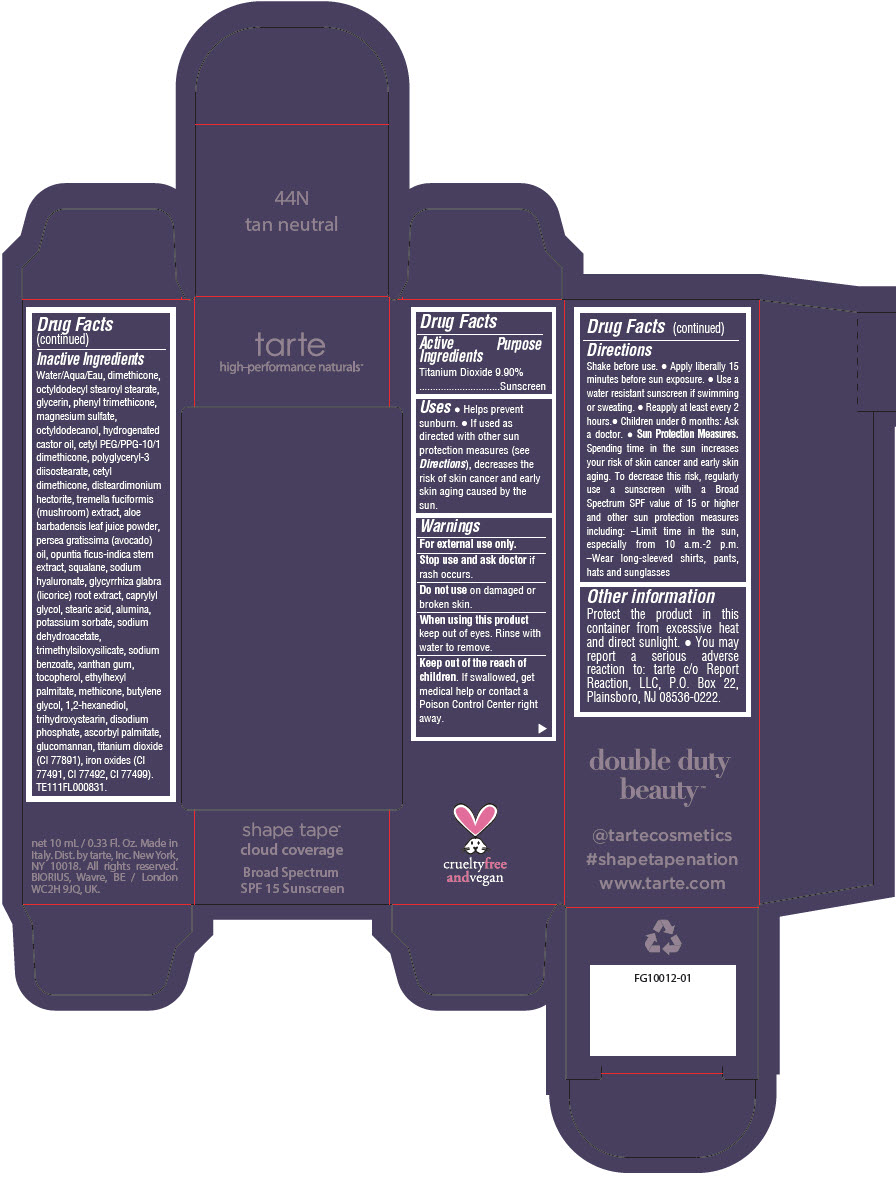
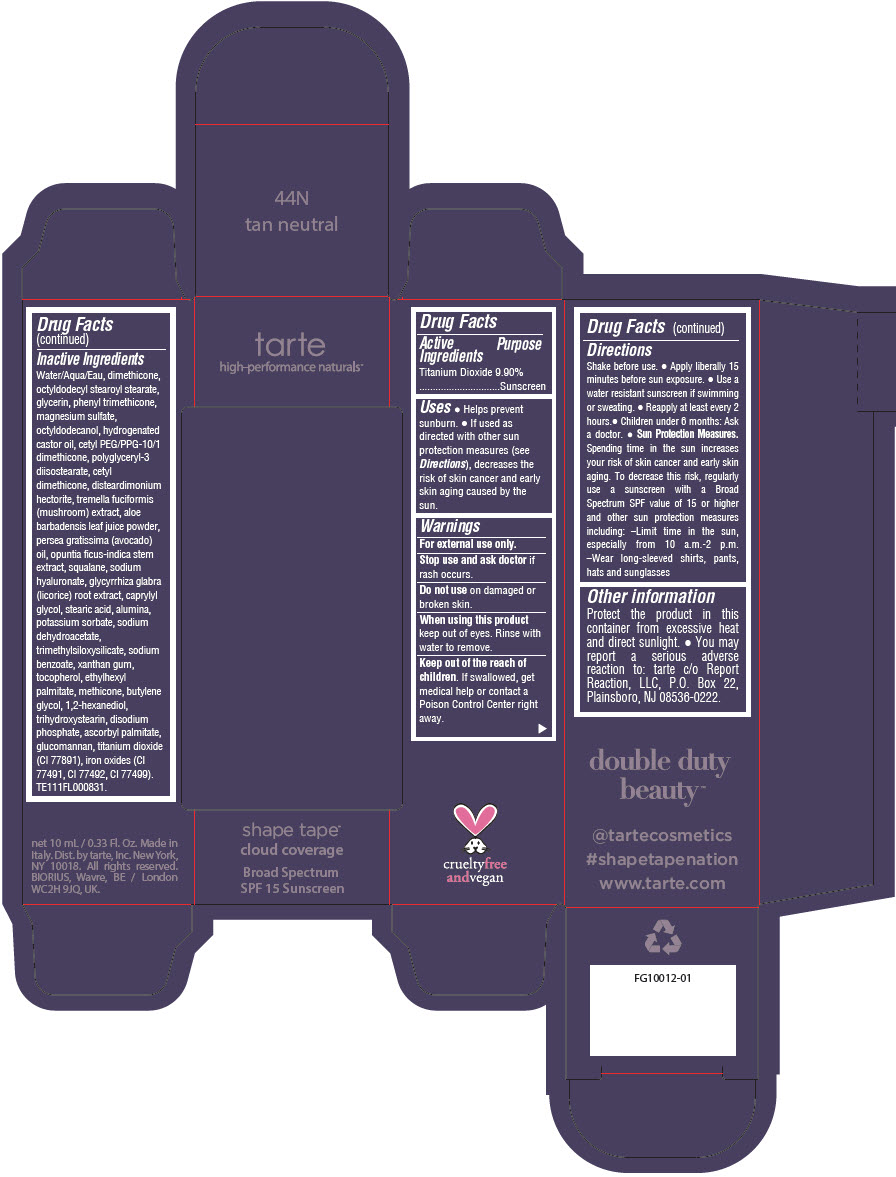
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 44N TAN NEUTRAL- titanium dioxide liquid
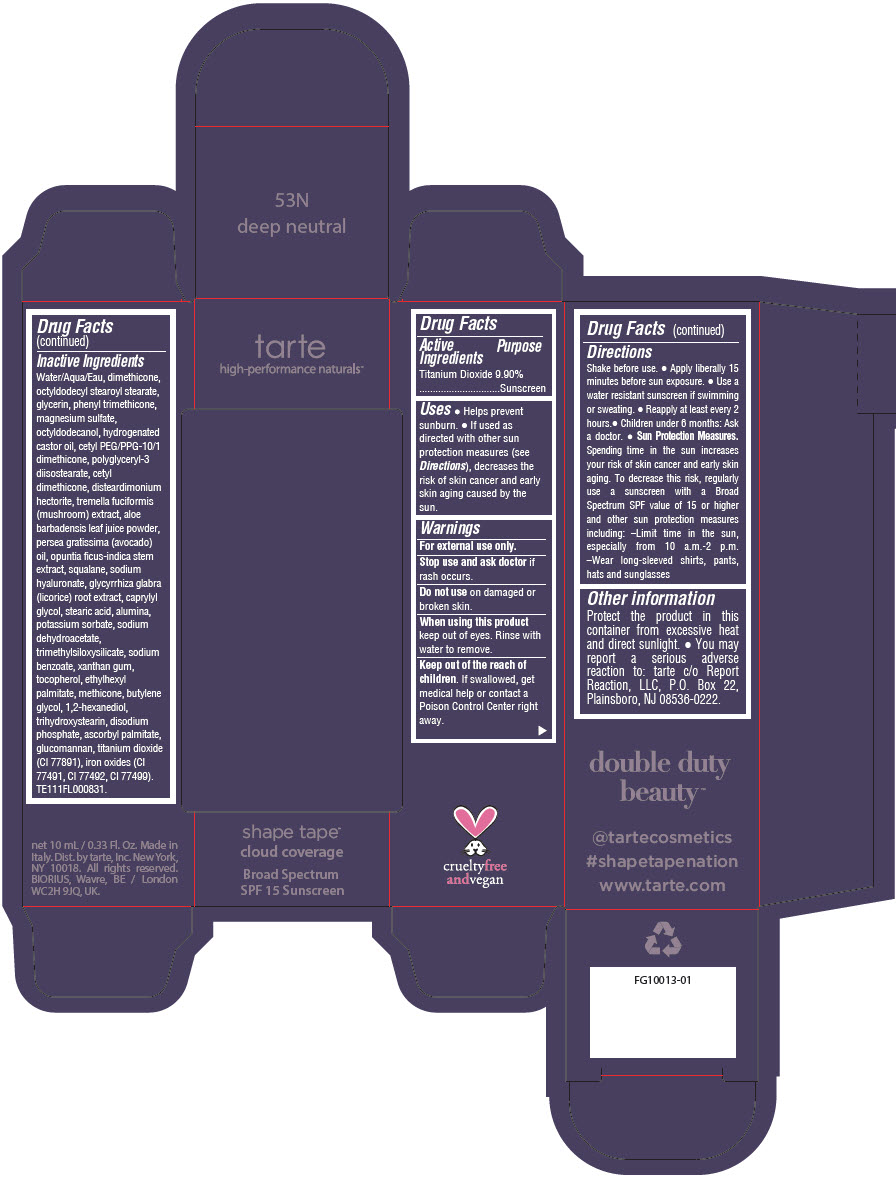
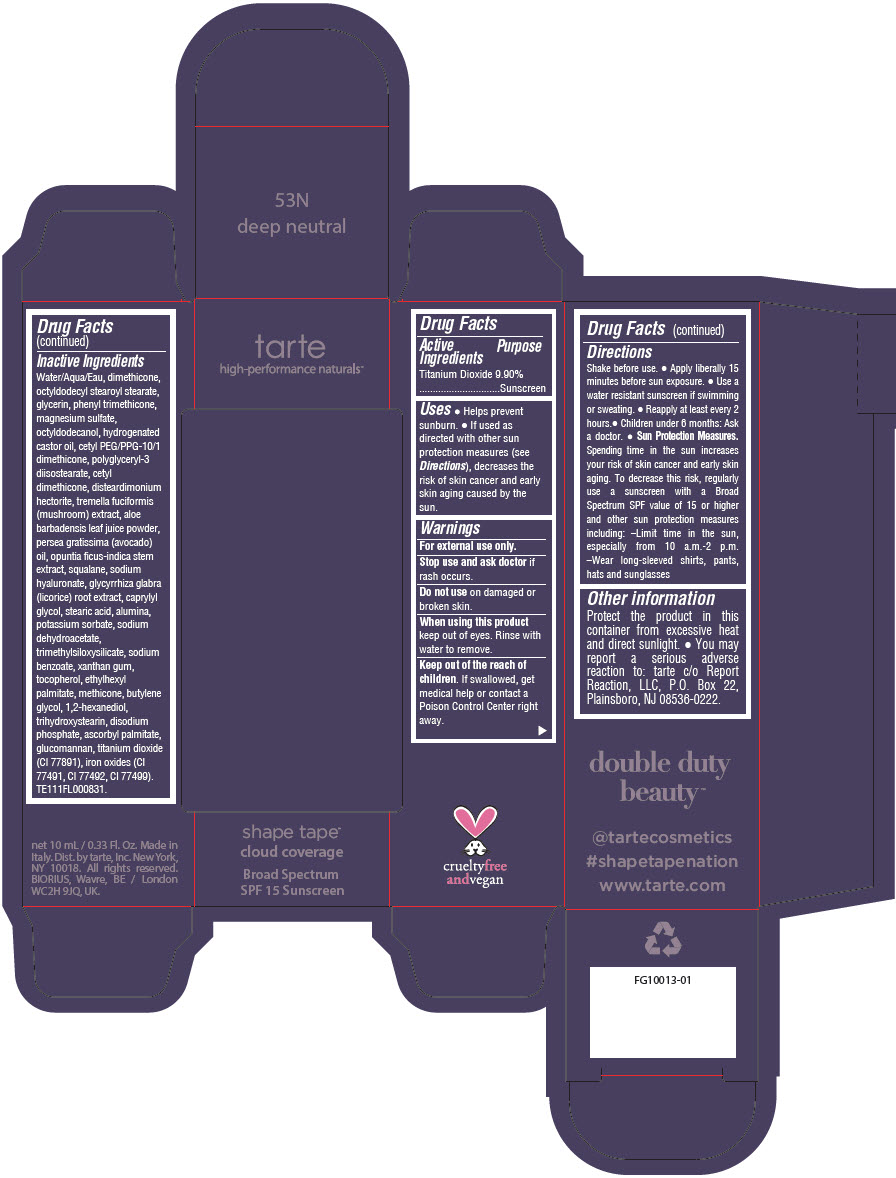
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 53N DEEP NEUTRAL- titanium dioxide liquid
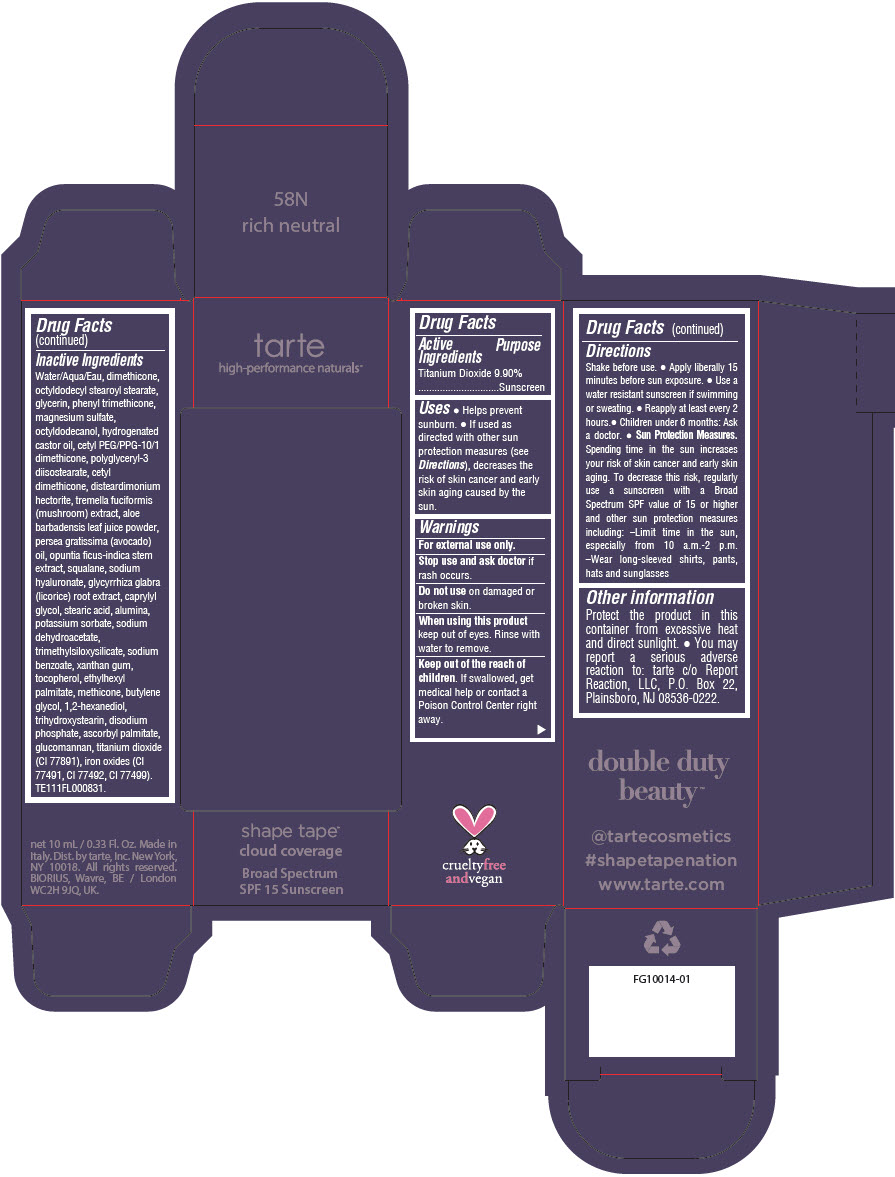
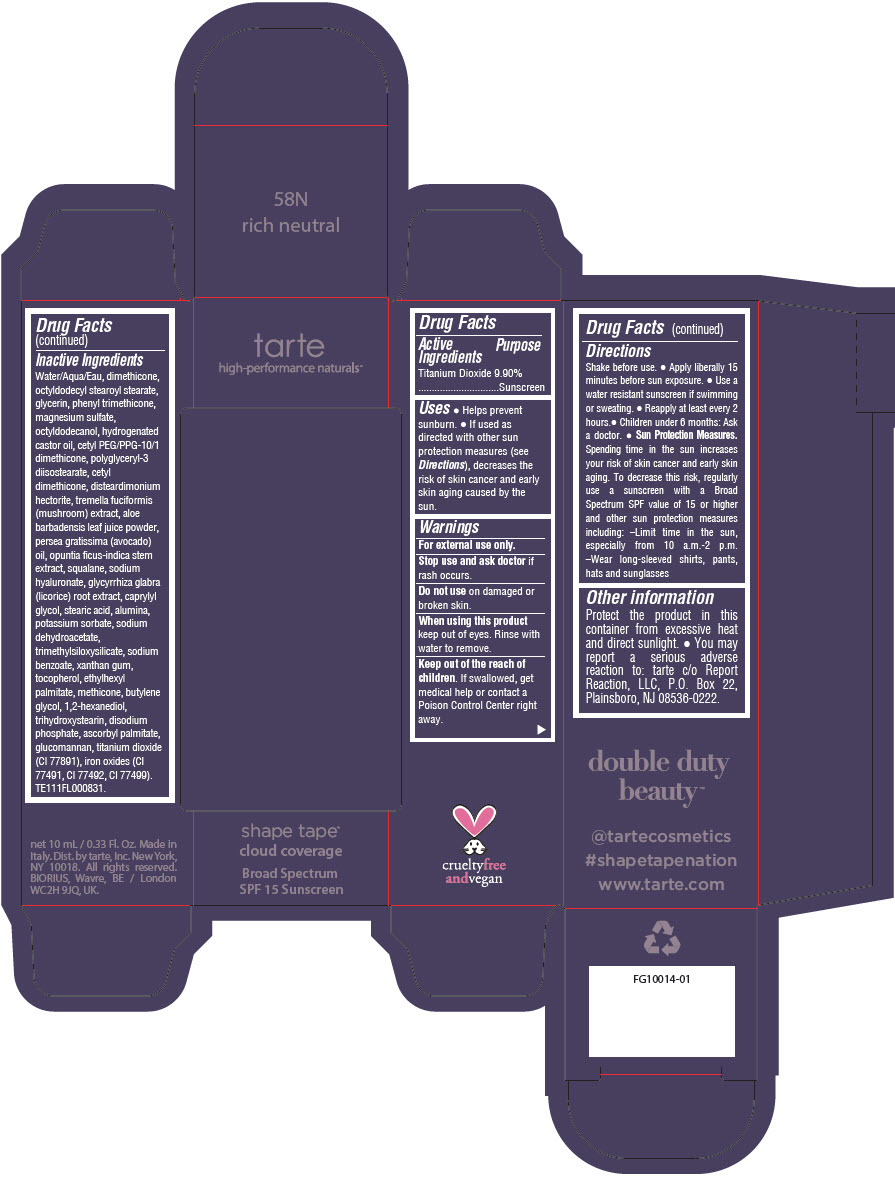
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 58N RICH NEUTRAL- titanium dioxide liquid
-
NDC Code(s):
51060-352-01,
51060-353-01,
51060-354-01,
51060-355-01, view more51060-356-01, 51060-357-01
- Packager: Tarte, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake before use.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive Ingredients
Water/Aqua/Eau, dimethicone, octyldodecyl stearoyl stearate, glycerin, phenyl trimethicone, magnesium sulfate, octyldodecanol, hydrogenated castor oil, cetyl PEG/PPG-10/1 dimethicone, polyglyceryl-3 diisostearate, cetyl dimethicone, disteardimonium hectorite, tremella fuciformis (mushroom) extract, aloe barbadensis leaf juice powder, persea gratissima (avocado) oil, opuntia ficus-indica stem extract, squalane, sodium hyaluronate, glycyrrhiza glabra (licorice) root extract, caprylyl glycol, stearic acid, alumina, potassium sorbate, sodium dehydroacetate, trimethylsiloxysilicate, sodium benzoate, xanthan gum, tocopherol, ethylhexyl palmitate, methicone, butylene glycol, 1,2-hexanediol, trihydroxystearin, sodium hyaluronate, disodium phosphate, ascorbyl palmitate, glucomannan, titanium dioxide (CI 77891), iron oxides (CI 77491, CI 77492, CI 77499). TE111FL000831.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 16N Fair-Light Neutral
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 22N Light Neutral
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 35N Medium Neutral
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 44N Tan Neutral
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 53N Deep Neutral
- PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton - 58N Rich Neutral
-
INGREDIENTS AND APPEARANCE
DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 16N FAIR-LIGHT NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-352 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-352-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 22N LIGHT NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-353 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-353-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 35N MEDIUM NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-354 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-354-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 44N TAN NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-355 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-355-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 53N DEEP NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-356 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-356-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 DOUBLE DUTY BEAUTY SHAPE TAPE CLOUD COVERAGE BROAD SPECTRUM SPF 15 SUNSCREEN 58N RICH NEUTRAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-357 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) OCTYLDODECANOL (UNII: 461N1O614Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LICORICE (UNII: 61ZBX54883) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYL PALMITATE (UNII: 2865993309) METHICONE (20 CST) (UNII: 6777U11MKT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ASCORBYL PALMITATE (UNII: QN83US2B0N) KONJAC MANNAN (UNII: 36W3E5TAMG) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-357-01 1 in 1 CARTON 11/26/2021 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/26/2021 Labeler - Tarte, Inc. (027905186)