Label: ONYCHO-MED- miconazole nitrate, terbinafine hcl kit
- NDC Code(s): 71150-786-10
- Packager: Medhart Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 16, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients:
- In-Active Ingredients:
-
How supplied:
Onycho-Med is a prescription only convenience kit for compounding by a healthcare professional for topical application in management of onychomycosis. The package contains premeasured bottles of Miconazole Nitrate 2% solution * and Terbinafine Hcl USP 250 mg** to make a final volume of 10 ml solution of Miconazole Nitrate 2% and Terbinafine Hcl USP 2.5%.
-
Indication:
Prescribed for the management of onychomycosis
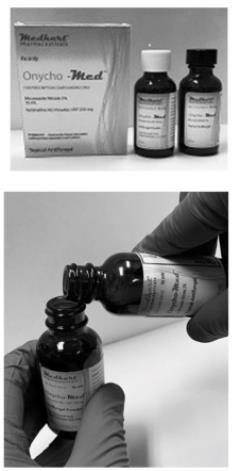
Mixing Instructions - Easy Two Step Process
- Please check expiration dates listed on the bottles
- Inspect product integrity seals on the bottles inside the carton
- Take the bottles out of the carton and set aside the carton, as illustrated in the picture to the left
- Open the black brush-cap lid on the Miconazole Nitrate 2% bottle and place it aside for re-use
- Carefully un-screw the white cap from the Terbinafine Hcl 250mg bottle and set aside to discard later
- Carefully pour the contents of the bottle labelled Miconazole Nitrate 2% into the bottle labelled Terbinafine Hcl powder 250mg
- Place the black brush-cap lid on the Terbinafine Hcl 250mg bottle, that now has Miconazole Nitrate 2% in it as well and tighten the lid
To the licensed medical professionals:
“This convenience kit is for prescription compounding only”
- Now shake the bottle containing Terbinafine Hcl 250mg USP powder and Miconazole Nitrate 2% solution vigorously for 30 seconds
- Make sure the contents have dissolved and there is no residue
- Apply the pharmacy label to the bottle with final solution
- Place the final solution (bottle with black brush cap) back into the carton
- Discard the empty bottle of Miconazole Nitrate 2% and the white cap appropriately
- Please instruct the patient to “shake well before use”

The non-compounded kit has a shelf-life of two years, The final product once mixed will follow a 30 day expiration as per USP 795 BUD (Beyond Use Date) guidelines. Healthcare professionals please follow your local state board of pharmacy BUD guidelines as well. Please discard any unused components of the kit appropriately. Miconazole Nitrate 2% solution meets the requirements for total aerobic microbial count of no more than 100 cfu/gm, as well as for absence of the specified microorganisms Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella and Candida albicans.
- Please check expiration dates listed on the bottles
-
Storage:
The convenience kit and the compounded product can be stored at room temperature between (15 – 30 0c) and after compounding should be discarded after 30 days as per USP 795 guidelines. Protect from light, protect from freezing.
Onycho-Med compounding kit components have a two-year expiration date ***
For External Use Only: Keep container tightly closed, keep out of reach of children, and avoid contact with eyes.
* This product is not manufactured by Valeant Pharma manufacturers of Fungoid tincture
** Certificate of Analysis on file
***Data and documentation on file
Users are encouraged to report adverse events to FDA MedWatch
1-800-FDA-1088 or at http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053074.htm
RX Only
Revised: April2019
Dear professionals we always value your feedback, please email at: suggestions@medhartpharma.com
Manufactured for:
Medhart Pharmaceuticals, Inc.
50 Eagle Rock Way, Suite C, Brentwood Ca 94513
Ph: 1-833-MEDHART (633-4278)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONYCHO-MED
miconazole nitrate, terbinafine hcl kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71150-786 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71150-786-10 1 in 1 BOX; Type 0: Not a Combination Product 05/30/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 25 BOTTLE 250 mL in 10 Part 2 1 BOTTLE, WITH APPLICATOR 10 mL Part 1 of 2 TERBINAFINE HYDROCHLORIDE
terbinafine hydrochloride solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERBINAFINE HYDROCHLORIDE (UNII: 012C11ZU6G) (TERBINAFINE - UNII:G7RIW8S0XP) TERBINAFINE HYDROCHLORIDE 250 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/30/2019 Part 2 of 2 MICONAZOLE NITRATE
miconazole nitrate solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 200 mg in 10 mL Inactive Ingredients Ingredient Name Strength RIBOPRINE (UNII: 8EU82FAZ5J) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) LAURETH-4 (UNII: 6HQ855798J) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/30/2019 Labeler - Medhart Pharmaceuticals, Inc. (080145788)


