Thank you for choosing Onycho-Med by Medhart Pharmaceuticals, Inc.
To the licensed medical professionals:
“This convenience kit is for prescription compounding only”
Please make sure the kit contains the following components:
Active Ingredients:
One Miconazole Nitrate USP 2% solution in 10ml bottle fitted with a brush cap lid One bottle containing 250 mgs of Terbinafine Hcl USP powder
In-Active Ingredients:
Acetic Acid, Aqua, Benzyl Alcohol, DMSO, IPA 70%, Laureth-4, PEG-6, Tea Tree Oil
How supplied:
Onycho-Med is a prescription only convenience kit for compounding by a healthcare professional for topical application in management of onychomycosis. The package contains premeasured bottles of Miconazole Nitrate 2% solution * and Terbinafine Hcl USP 250 mg** to make a final volume of 10 ml solution of Miconazole Nitrate 2% and Terbinafine Hcl USP 2.5%.
Indication:
Prescribed for the management of onychomycosis
Mixing Instructions - Easy Two Step Process
- Please check expiration dates listed on the bottles
- Inspect product integrity seals on the bottles inside the carton
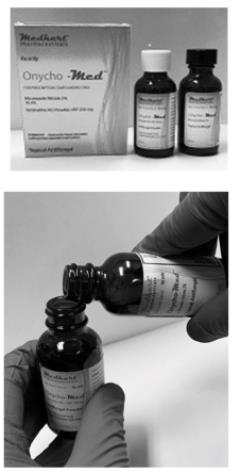
- Take the bottles out of the carton and set aside the carton, as illustrated in the picture to the left
- Open the black brush-cap lid on the Miconazole Nitrate 2% bottle and place it aside for re-use
- Carefully un-screw the white cap from the Terbinafine Hcl 250mg bottle and set aside to discard later
- Carefully pour the contents of the bottle labelled Miconazole Nitrate 2% into the bottle labelled Terbinafine Hcl powder 250mg
- Place the black brush-cap lid on the Terbinafine Hcl 250mg bottle, that now has Miconazole Nitrate 2% in it as well and tighten the lid
To the licensed medical professionals:
“This convenience kit is for prescription compounding only”
- Now shake the bottle containing Terbinafine Hcl 250mg USP powder and Miconazole Nitrate 2% solution vigorously for 30 seconds
- Make sure the contents have dissolved and there is no residue
- Apply the pharmacy label to the bottle with final solution
- Place the final solution (bottle with black brush cap) back into the carton
- Discard the empty bottle of Miconazole Nitrate 2% and the white cap appropriately
- Please instruct the patient to “shake well before use”

The non-compounded kit has a shelf-life of two years, The final product once mixed will follow a 30 day expiration as per USP 795 BUD (Beyond Use Date) guidelines. Healthcare professionals please follow your local state board of pharmacy BUD guidelines as well. Please discard any unused components of the kit appropriately. Miconazole Nitrate 2% solution meets the requirements for total aerobic microbial count of no more than 100 cfu/gm, as well as for absence of the specified microorganisms Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella and Candida albicans.
Storage:
The convenience kit and the compounded product can be stored at room temperature between (15 – 30 0c) and after compounding should be discarded after 30 days as per USP 795 guidelines. Protect from light, protect from freezing.
Onycho-Med compounding kit components have a two-year expiration date ***
For External Use Only: Keep container tightly closed, keep out of reach of children, and avoid contact with eyes.
* This product is not manufactured by Valeant Pharma manufacturers of Fungoid tincture
** Certificate of Analysis on file
***Data and documentation on file
Users are encouraged to report adverse events to FDA MedWatch
1-800-FDA-1088 or at http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053074.htm
RX Only
Revised: April2019
Dear professionals we always value your feedback, please email at: suggestions@medhartpharma.com
Manufactured for:
Medhart Pharmaceuticals, Inc.
50 Eagle Rock Way, Suite C, Brentwood Ca 94513
Ph: 1-833-MEDHART (633-4278)
