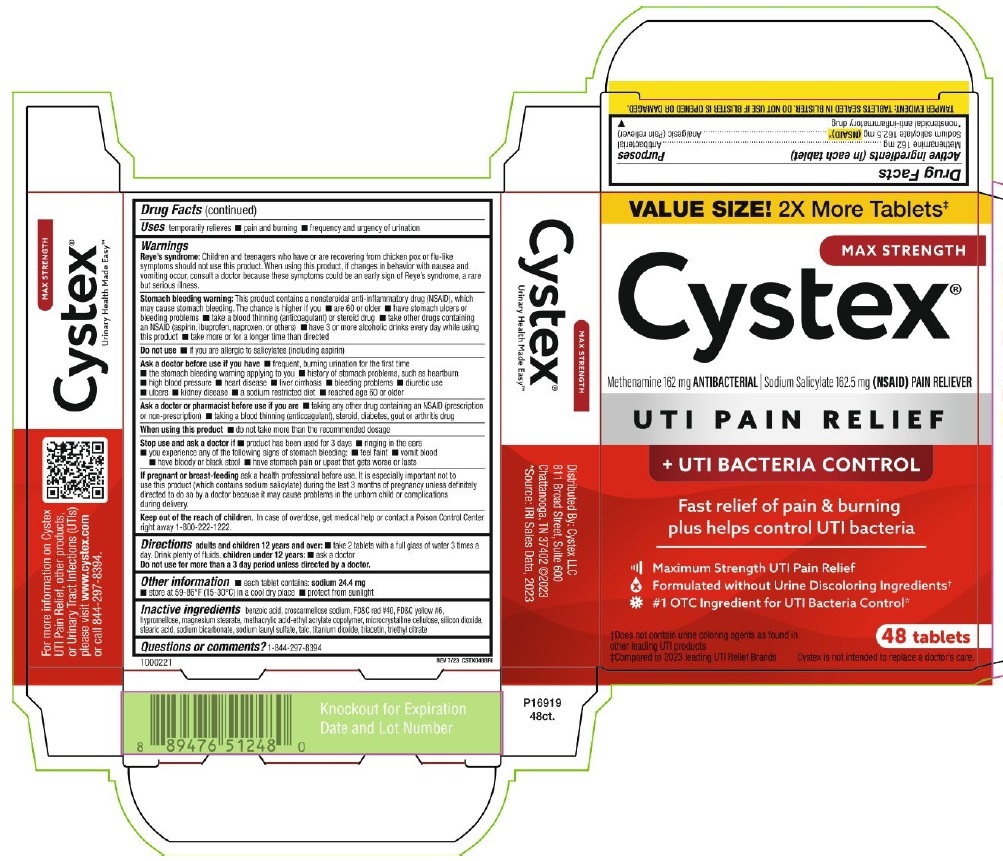
Label: CYSTEX PLUS- methenamine, sodium salicylate tablet
- NDC Code(s): 69693-512-20, 69693-512-24, 69693-512-40, 69693-512-48
- Packager: Clarion Brands, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 22, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you
- are 60 or older
- have stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
-
PREGNANCY OR BREAST FEEDING
If pregnant or breast feeding ask a health professional before use. It is especially important not to use this product (which contains sodium salicylate) during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
benzoic acid, croscarmellose sodium, fd&c red #40, fd&c yellow #6, hypromellose , magnesium stearate, methacrylic acid-ethyl acrylate copolymer, microcrystalline cellulose, silicon dioxide, stearic acid, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl titanium dioxide, triethyl citrate
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
VALUE SIZE! 2x More Tablets‡
MAX STRENGTH
Cystex®
Methenamine 162 mg ANTIBACTERIAL ∣ Sodium Salicylate 162.5 mg (NSAID) PAIN RELIEVER
UTI PAIN RELIEF
+ UTI BACTERIA CONTROL
Fast relief of pain & burning
plus helps control UTI bacteria
Maximum Strength UTI Pain Relief
Formulated without Urine Discoloring Ingredients†
#1 OTC Ingredient for UTI Bacteria Control*
†Does not contain urine coloring agents as found in
other leading UTI products
‡Compared to 2023 leading UTI Relief Brands
48 Tablets
Cystex is not intended to replace a doctor's care.
TAMPER EVIDENT: TABLETS SEALED IN BLISTER. DO NOT USE IF BLISTER IS OPENED OR DAMAGED.
1000221
REV 7/23 CSTX048BRI
MAX STRENGTH
Cystex®
Urinary Health Made Easy™
For more information on Cystex
UTI Pain Relief, other products,
or Urinary Tract Infections (UTIs)
please visit www.cyxtex.com
or call 844-297-8394
MAX STRENGTH
Cystex®
Urinary Health Made Easy™
Distributed by: Cystex, LLC
811 Broad Street, Suite 600
Chattanooga, TN 37402 @2023
*Source: IRI Sales Data, 2023
P16919
48ct.
Knockout for Expiration
Date and Lot Number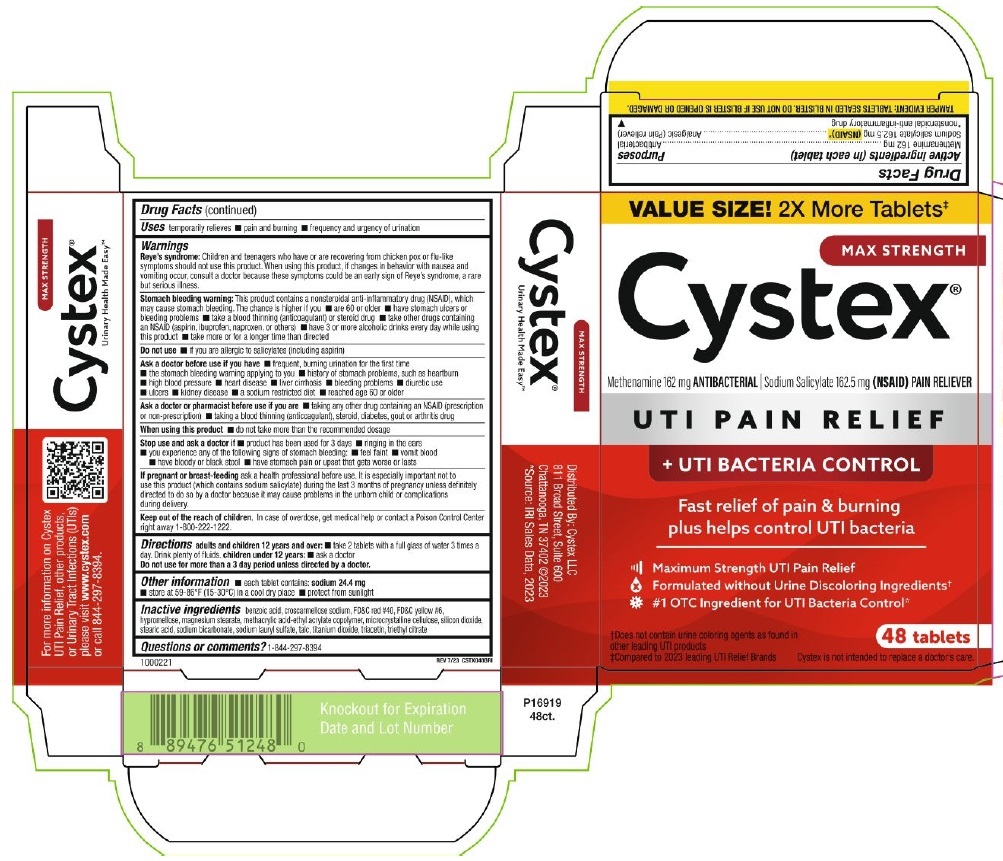
Principal Display for Blister Pack
CYSTEX
Methenamine 162 mg / Sodium Salicylate 162.5 mg (NSAID)
URINARY PAIN RELIEF TABLETS
Reye's Syndrome: Children and teenagers who have or are recovering
from chicken pox or flu-like symptoms should not use this product. When
using this product, if changes in behavior with nausea and vomiting occur,
consult a doctor because these symptoms could be an early sign of Reye's
syndrome, a rare but serious illness. Stomach Bleeding Warning: This
product contains a nonsteroidal anti-inflammatory drug (NSAID), which
may cause stomach bleeding. The chance is higher if you: ■ are age 60 or
older ■ have stomach ulcers or bleeding problems ■ take a blood thinner
(anticoagulant) or steroid drug ■ take other drugs containing an NSAID
(aspirin, ibuprofen, naproxen, or others) ■ have 3 or more alcoholic drinks
every day while using this product ■ take more or for a longer time
than directed.
ATTENTION: USE ONLY IF TABLET
BLISTER SEALS ARE UNBROKEN
LOT: X00X000 EXP: MM/YY
CYSTEX, LLC. 1-844-297-8394
1000075
-
INGREDIENTS AND APPEARANCE
CYSTEX PLUS
methenamine, sodium salicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69693-512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methenamine (UNII: J50OIX95QV) (Methenamine - UNII:J50OIX95QV) Methenamine 162 mg Sodium Salicylate (UNII: WIQ1H85SYP) (Salicylic Acid - UNII:O414PZ4LPZ) Sodium Salicylate 162.5 mg Inactive Ingredients Ingredient Name Strength Benzoic Acid (UNII: 8SKN0B0MIM) Croscarmellose Sodium (UNII: M28OL1HH48) Fd&c Red No. 40 (UNII: WZB9127XOA) Fd&c Yellow No. 6 (UNII: H77VEI93A8) Hypromellose, Unspecified (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) Methacrylic Acid And Ethyl Acrylate Copolymer (UNII: NX76LV5T8J) Microcrystalline Cellulose (UNII: OP1R32D61U) Silicon Dioxide (UNII: ETJ7Z6XBU4) Stearic Acid (UNII: 4ELV7Z65AP) Sodium Bicarbonate (UNII: 8MDF5V39QO) Sodium Lauryl Sulfate (UNII: 368GB5141J) Talc (UNII: 7SEV7J4R1U) Titanium Dioxide (UNII: 15FIX9V2JP) Triacetin (UNII: XHX3C3X673) Triethyl Citrate (UNII: 8Z96QXD6UM) Product Characteristics Color RED Score no score Shape ROUND Size 10mm Flavor Imprint Code CYSTEX Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69693-512-40 1 in 1 CARTON 05/01/2016 1 40 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:69693-512-20 1 in 1 CARTON 05/01/2016 2 20 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:69693-512-24 1 in 1 CARTON 06/01/2022 3 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:69693-512-48 1 in 1 CARTON 06/01/2022 4 48 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/01/2015 Labeler - Clarion Brands, LLC (079742703) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture(69693-512)