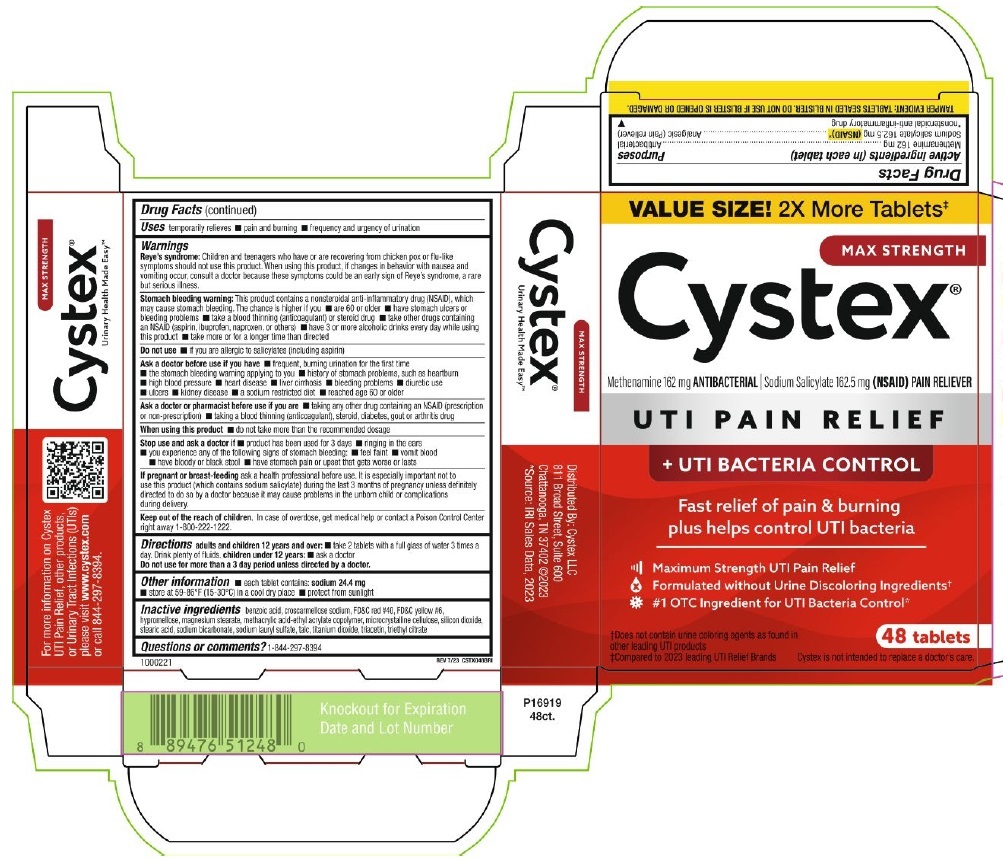
| Active ingredients (in each tablet) | Purposes |
| Methenamine 162 mg | Antibacterial |
| Sodium Salicylate 162.5 mg (NSAID)* | Analgesic (Pain Reliever) |
| * nonsteroidal anti-inflammatory drug |
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you
- are 60 or older
- have stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if you have
- frequent, burning urination for the first time
- the stomach bleeding warning applying to you
- history of stomach problems, such as heartburn
- high blood pressure
- heart disease
- liver cirrhosis
- bleeding problems
- diuretic use
- ulcers
- kidney disease
- a sodium restricted diet
- reach age 60 or older
Ask a doctor or pharmacist before use if you are
- taking any other drug containing an NSAID (prescription or non-prescription)
- taking a blood thinning (anticoagulant), steroid, diabetes, gout or arthritis drug
Stop use and ask a doctor if
- product has been used for 3 days
- ringing in the ears
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain or upset that gets worse or lasts
If pregnant or breast feeding ask a health professional before use. It is especially important not to use this product (which contains sodium salicylate) during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away 1-800-222-1222.
Directions
Adults and children 12 years and over:
- take 2 tablets with a full glass of water 3 times a day. Drink plenty of fluids.
Children under 12 years:
- ask a doctor.
Do not use for more than a 3 day period unless directed by a doctor.
Other information
- each tablet contains: sodium 24.4 mg
- store at 59-86°F (15-30°C) in a cool dry place
- protect from sunlight
Inactive ingredients
benzoic acid, croscarmellose sodium, fd&c red #40, fd&c yellow #6, hypromellose , magnesium stearate, methacrylic acid-ethyl acrylate copolymer, microcrystalline cellulose, silicon dioxide, stearic acid, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl titanium dioxide, triethyl citrate
PRINCIPAL DISPLAY PANEL
VALUE SIZE! 2x More Tablets‡
MAX STRENGTH
Cystex®
Methenamine 162 mg ANTIBACTERIAL ∣ Sodium Salicylate 162.5 mg (NSAID) PAIN RELIEVER
UTI PAIN RELIEF
+ UTI BACTERIA CONTROL
Fast relief of pain & burning
plus helps control UTI bacteria
Maximum Strength UTI Pain Relief
Formulated without Urine Discoloring Ingredients†
#1 OTC Ingredient for UTI Bacteria Control*
†Does not contain urine coloring agents as found in
other leading UTI products
‡Compared to 2023 leading UTI Relief Brands
48 Tablets
Cystex is not intended to replace a doctor's care.
TAMPER EVIDENT: TABLETS SEALED IN BLISTER. DO NOT USE IF BLISTER IS OPENED OR DAMAGED.
1000221
REV 7/23 CSTX048BRI
MAX STRENGTH
Cystex®
Urinary Health Made Easy™
For more information on Cystex
UTI Pain Relief, other products,
or Urinary Tract Infections (UTIs)
please visit www.cyxtex.com
or call 844-297-8394
MAX STRENGTH
Cystex®
Urinary Health Made Easy™
Distributed by: Cystex, LLC
811 Broad Street, Suite 600
Chattanooga, TN 37402 @2023
*Source: IRI Sales Data, 2023
P16919
48ct.
Knockout for Expiration
Date and Lot Number
Principal Display for Blister Pack
CYSTEX
Methenamine 162 mg / Sodium Salicylate 162.5 mg (NSAID)
URINARY PAIN RELIEF TABLETS
Reye's Syndrome: Children and teenagers who have or are recovering
from chicken pox or flu-like symptoms should not use this product. When
using this product, if changes in behavior with nausea and vomiting occur,
consult a doctor because these symptoms could be an early sign of Reye's
syndrome, a rare but serious illness. Stomach Bleeding Warning: This
product contains a nonsteroidal anti-inflammatory drug (NSAID), which
may cause stomach bleeding. The chance is higher if you: ■ are age 60 or
older ■ have stomach ulcers or bleeding problems ■ take a blood thinner
(anticoagulant) or steroid drug ■ take other drugs containing an NSAID
(aspirin, ibuprofen, naproxen, or others) ■ have 3 or more alcoholic drinks
every day while using this product ■ take more or for a longer time
than directed.
ATTENTION: USE ONLY IF TABLET
BLISTER SEALS ARE UNBROKEN
LOT: X00X000 EXP: MM/YY
CYSTEX, LLC. 1-844-297-8394
1000075
