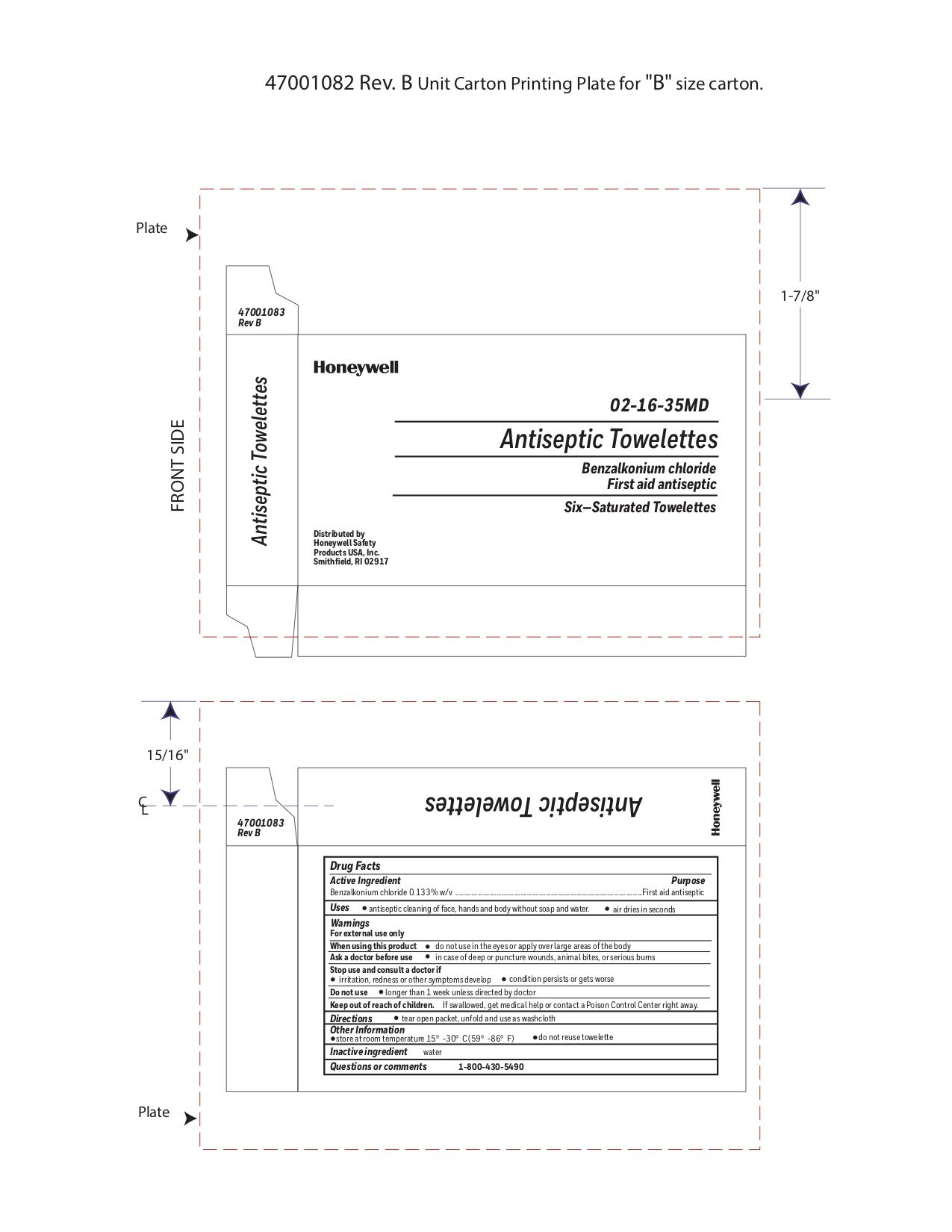
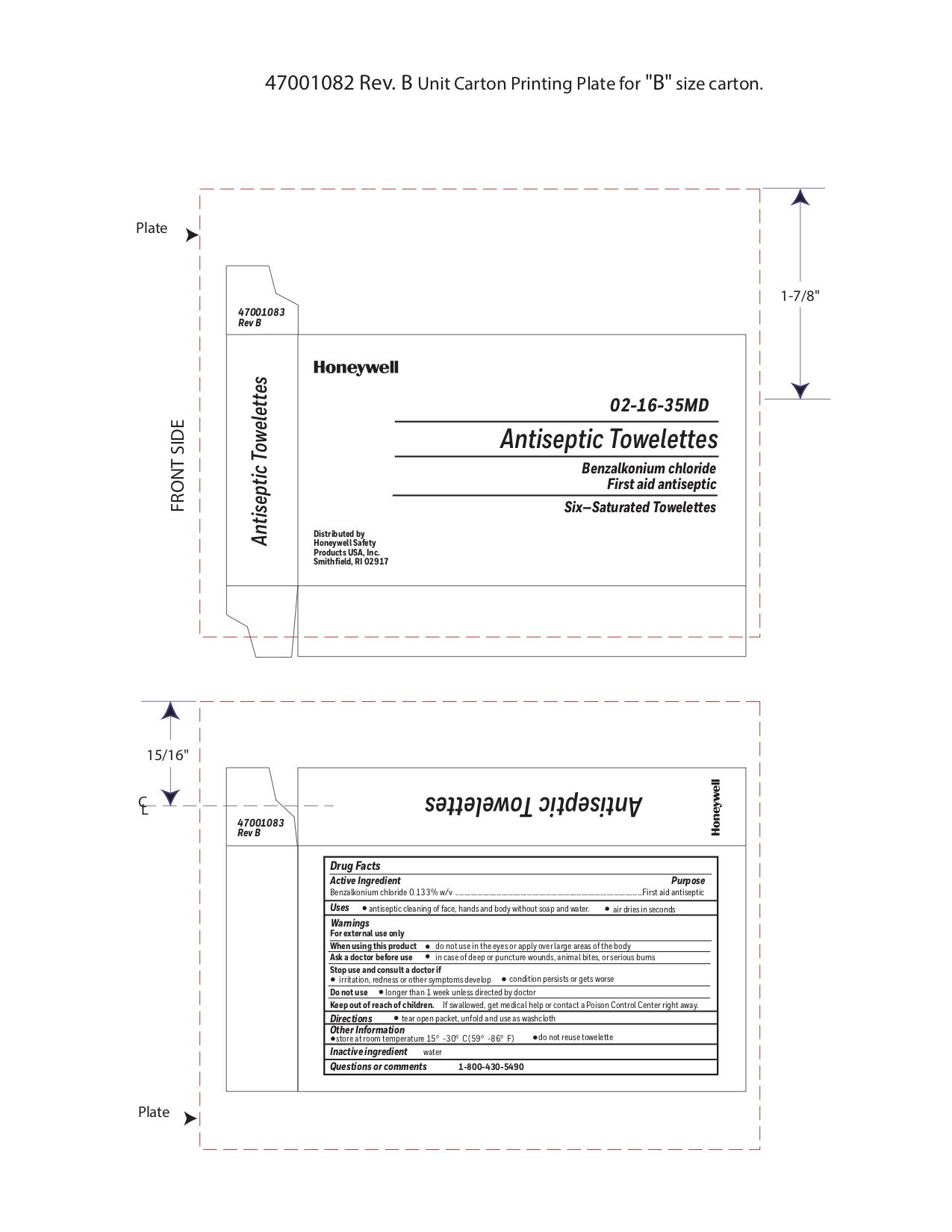
Label: ANTISEPTIC TOWELETTE- benzalkonium chloride liquid
- NDC Code(s): 0498-0501-00, 0498-0501-34, 0498-0501-37
- Packager: Honeywell Safety Products USA, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0501-34 10 in 1 BOX, UNIT-DOSE 12/21/2017 1 1.4 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:0498-0501-37 6 in 1 BOX, UNIT-DOSE 12/21/2017 2 1.4 mL in 1 PACKET; Type 0: Not a Combination Product 3 NDC:0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product 12/21/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/21/2017 Labeler - Honeywell Safety Products USA, Inc (118768815) Registrant - Honeywell Safety Products USA, Inc (118768815)