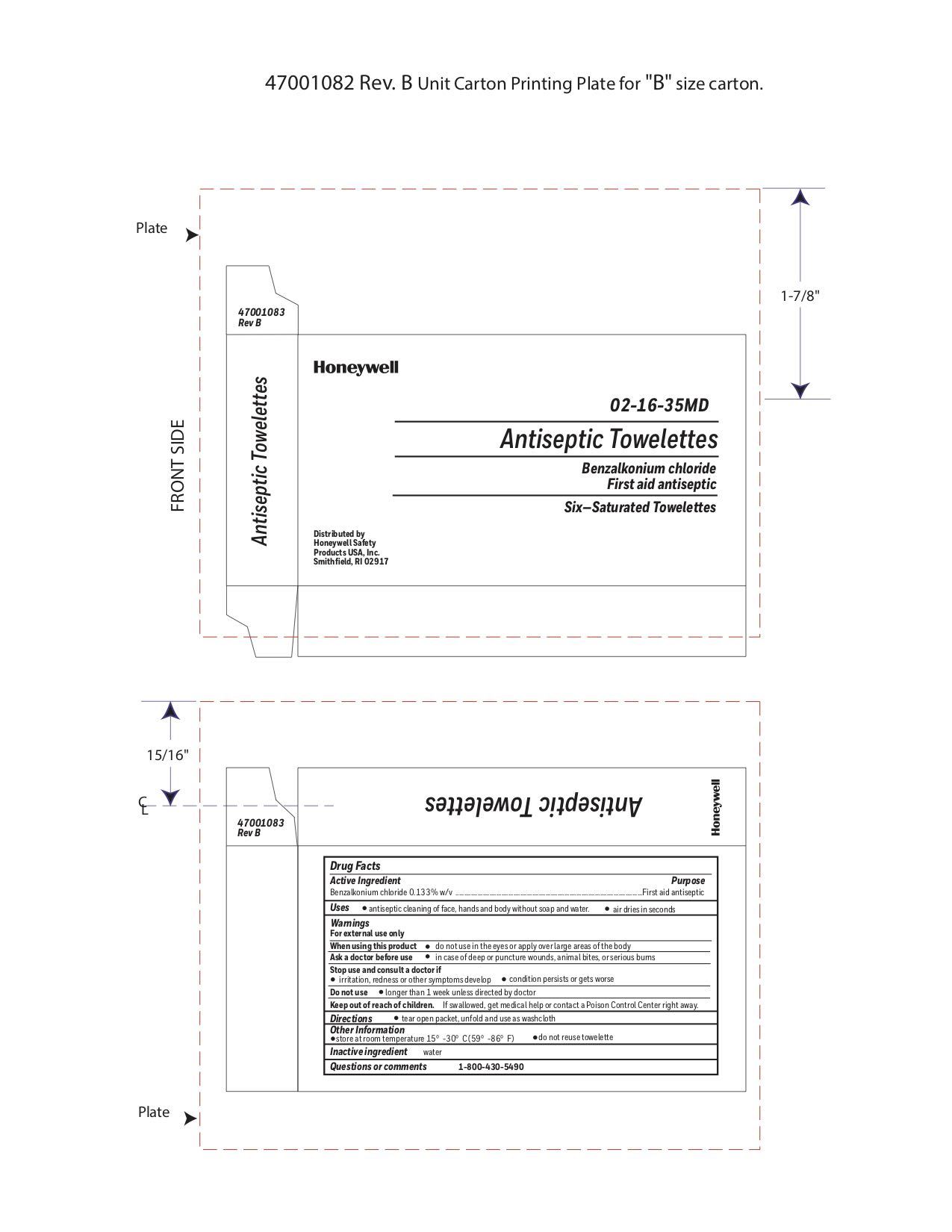
Active ingredient
Benzalkonium chloride 0.13% w/v
Purpose
First aid antiseptic
Uses
Antiseptic cleansing of face, hands, and body without soap and water
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- tear open packet and use as a washcloth
Other information
- store at room temperature 15
0 to 30
0C (59
0 - 86
0F)
- do not reuse towelette
Inactive ingredients
water
Principal Display Panel