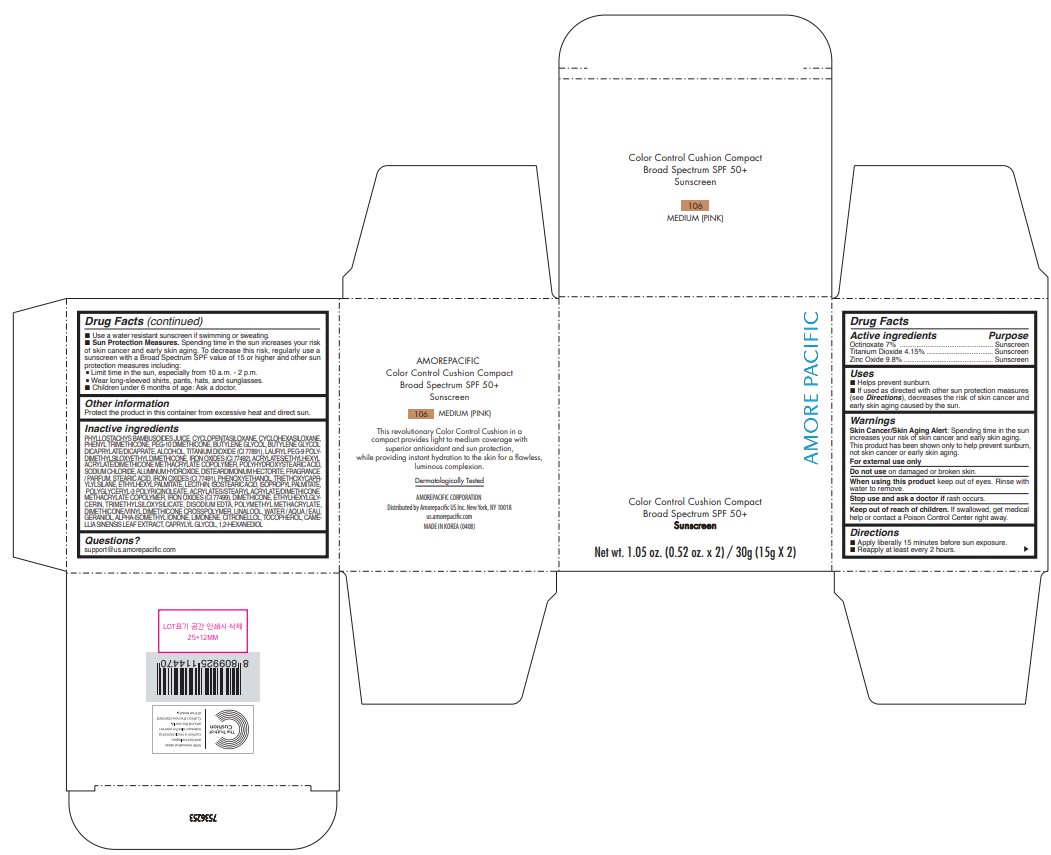
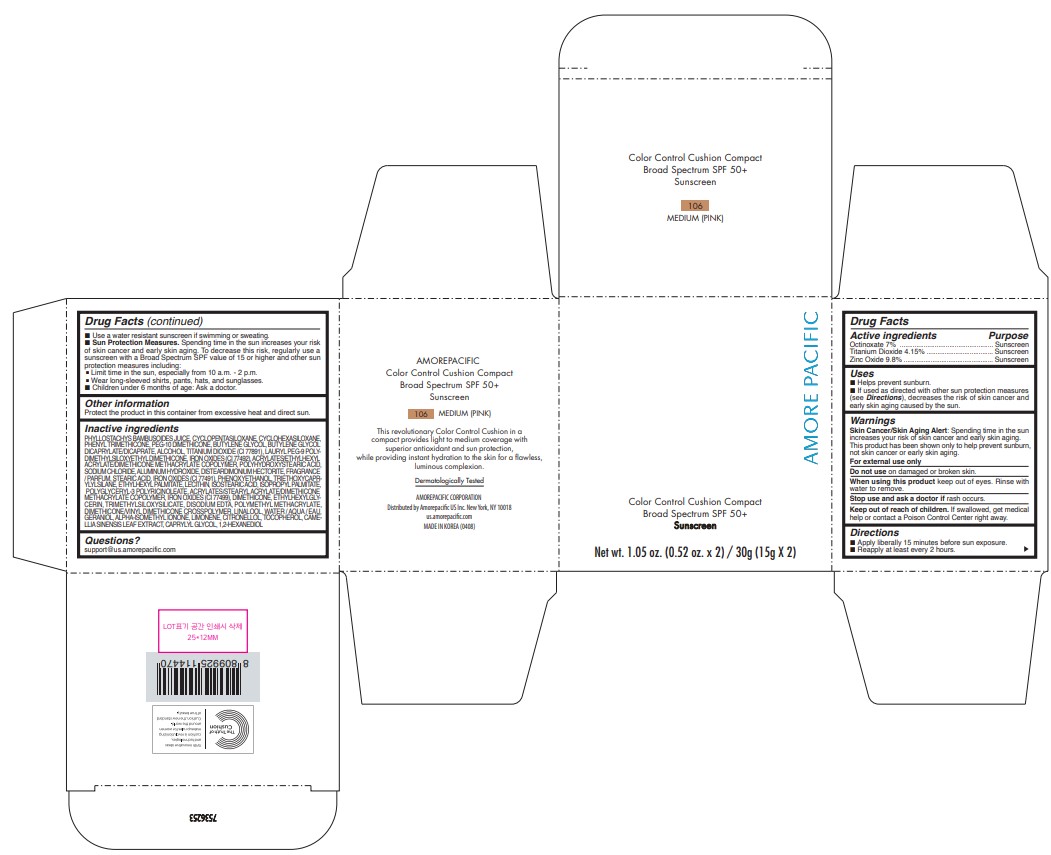
Label: AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.106- octinoxate, titanium dioxide, and zinc oxide lotion
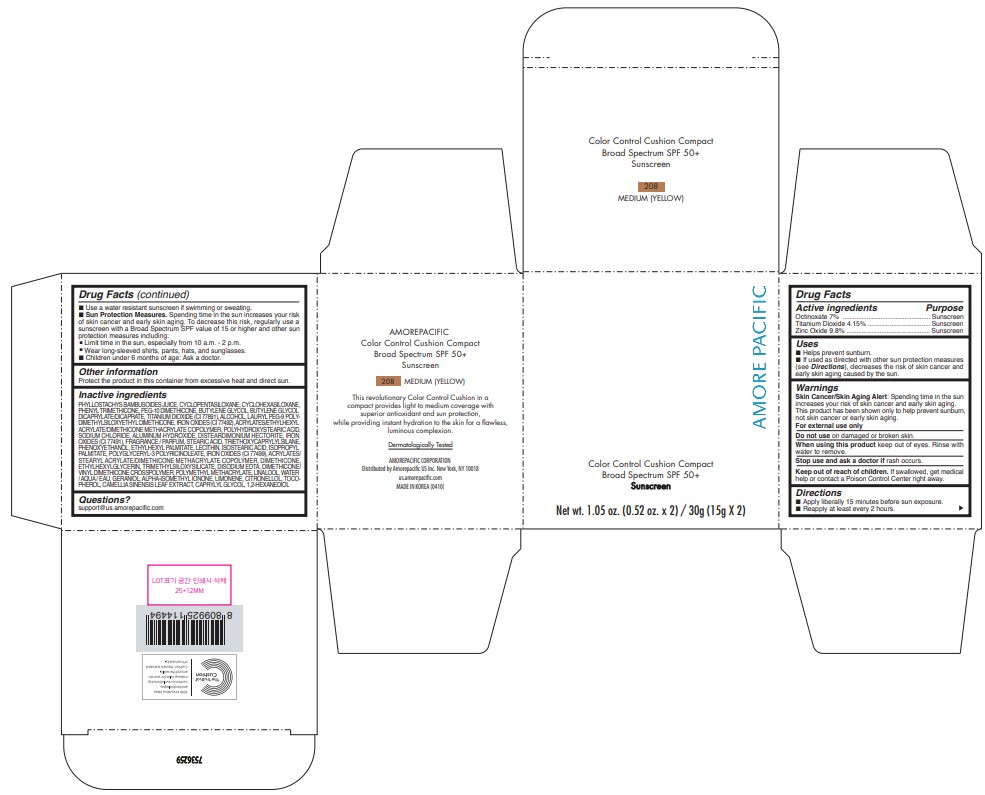
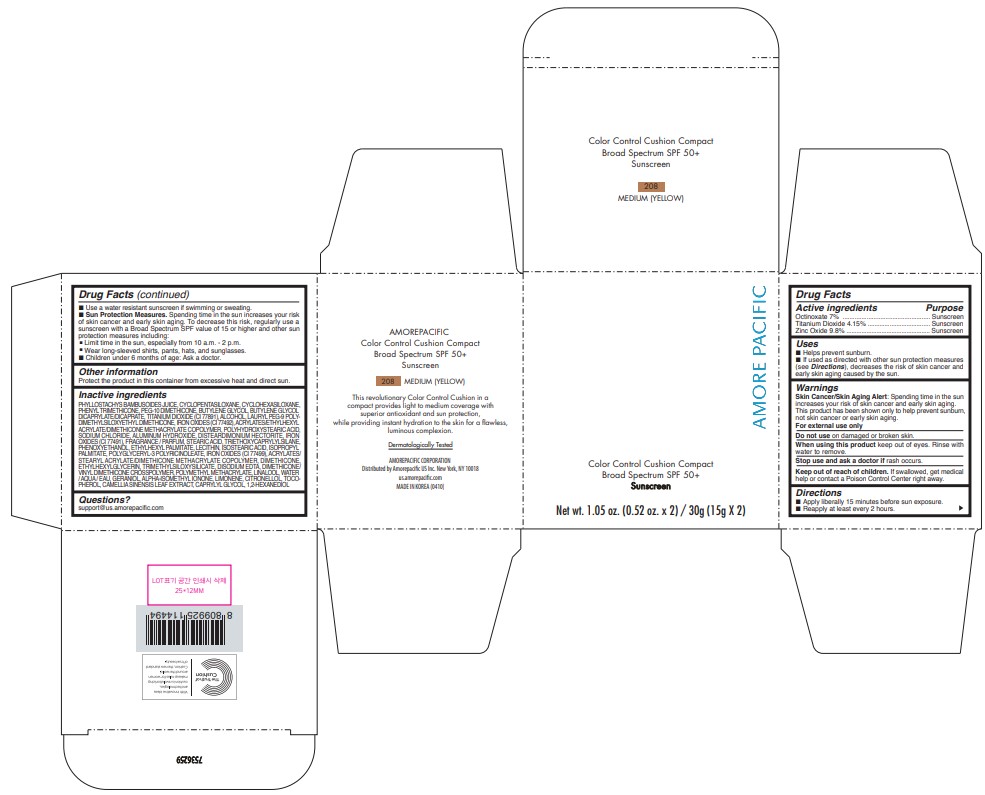
AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.208- octinoxate, titanium dioxide, and zinc oxide lotion
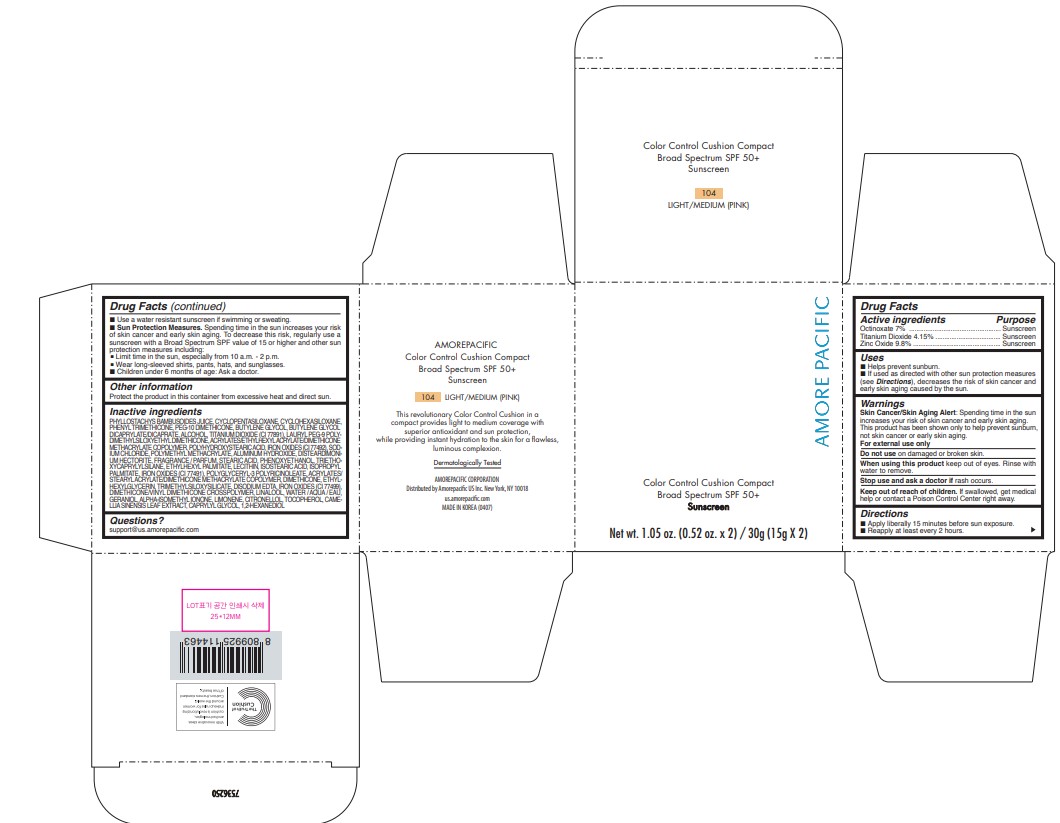
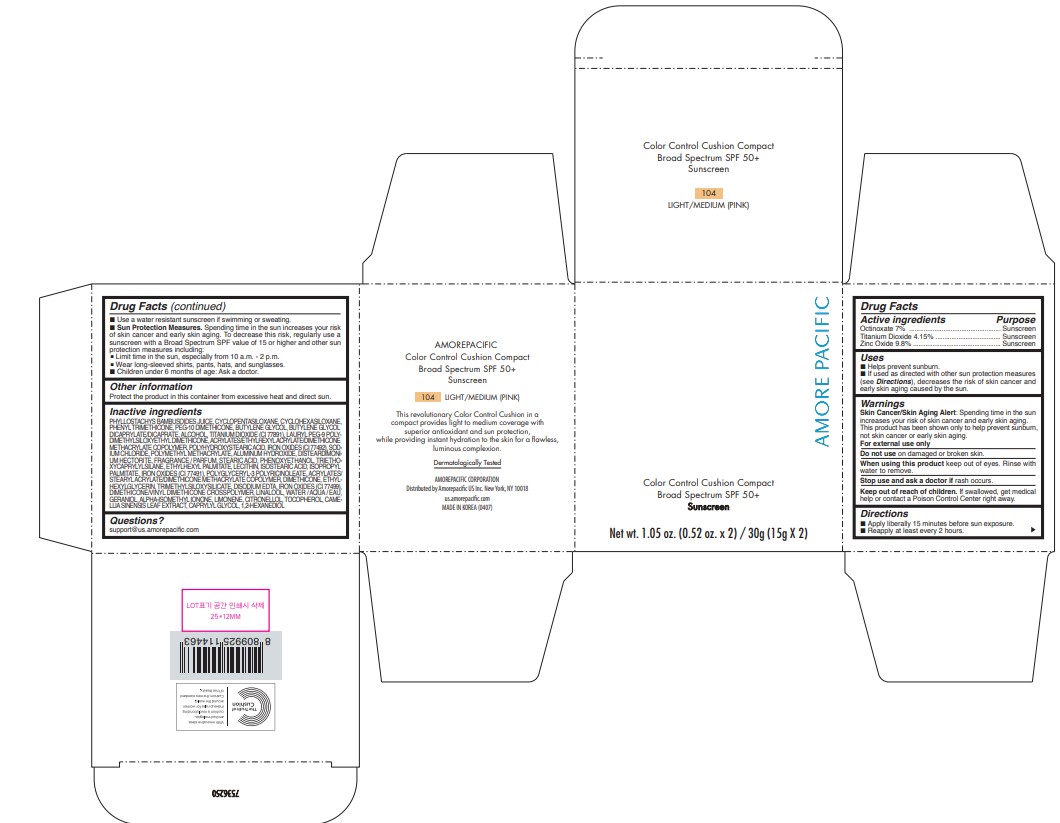
AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.104- octinoxate, titanium dioxide, and zinc oxide lotion
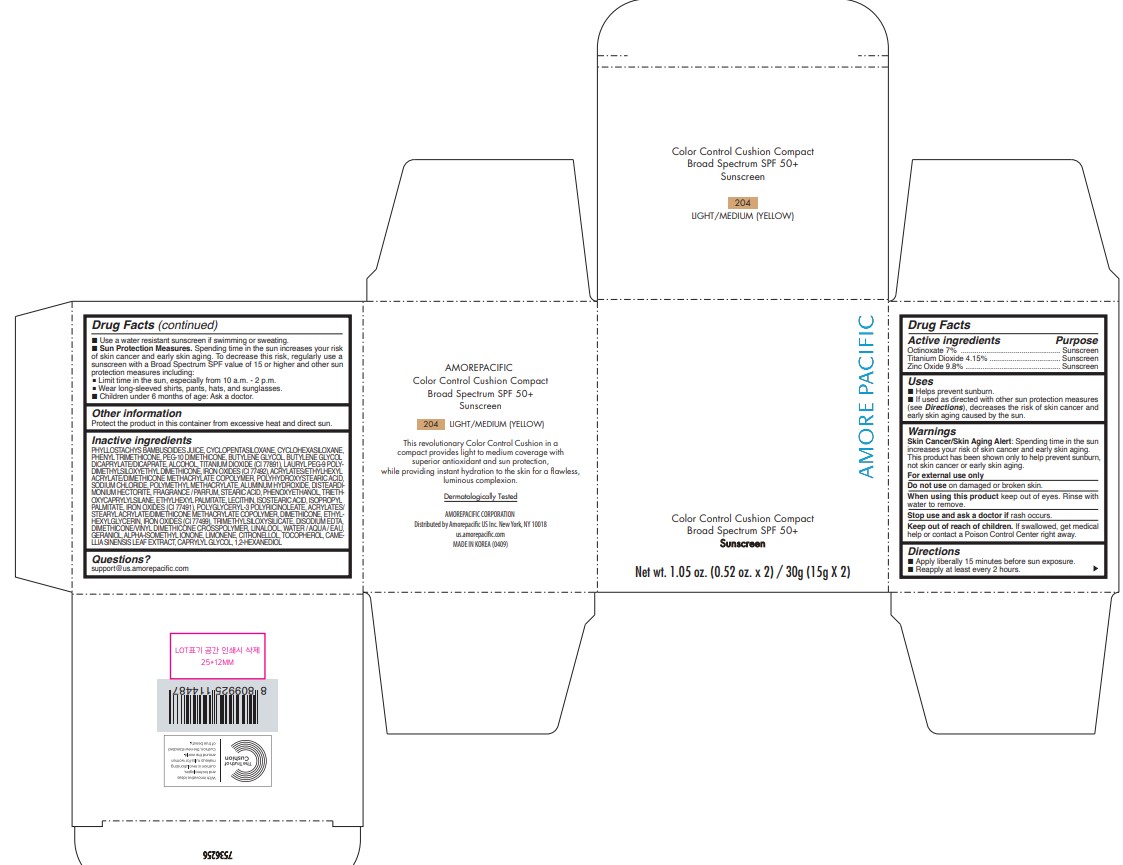
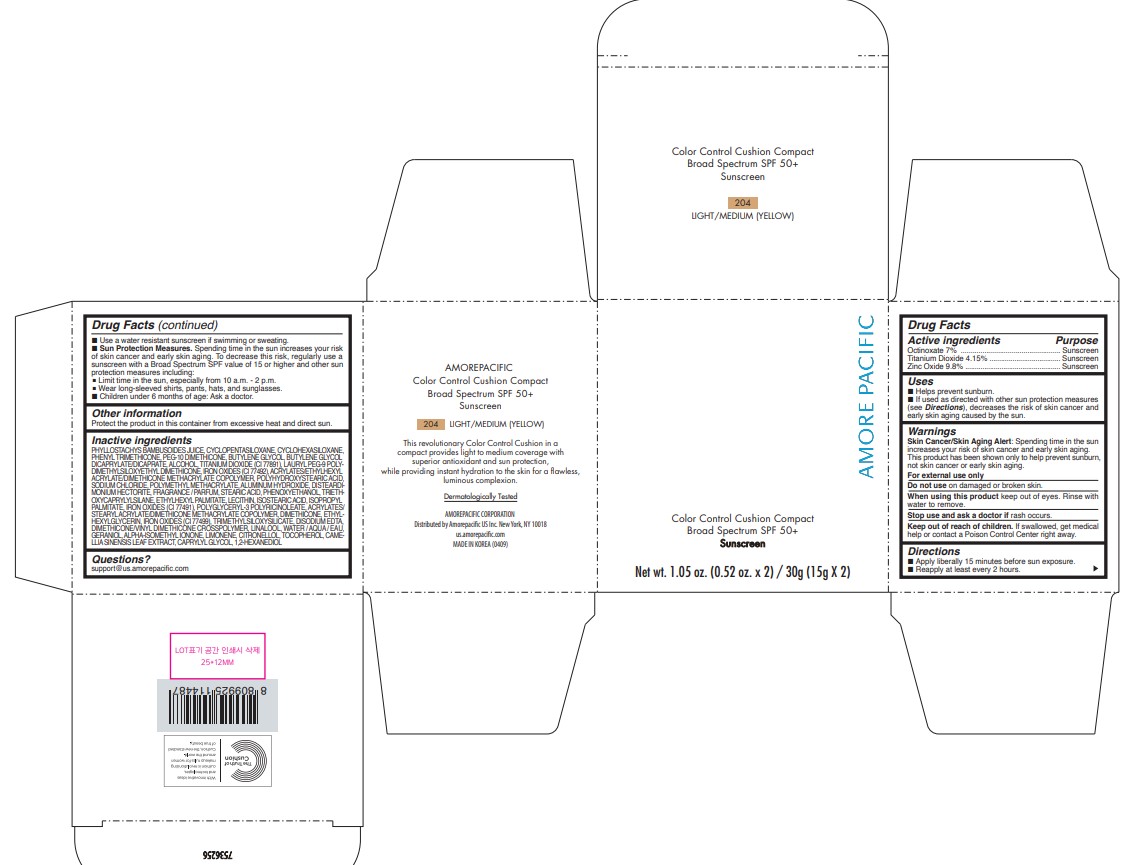
AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.204- octinoxate, titanium dioxide, and zinc oxide lotion
- NDC Code(s): 43419-511-21, 43419-512-21, 43419-513-21, 43419-514-21
- Packager: AMOREPACIFIC CORPORATION
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENTS
- PURPOSE
-
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- WARNINGS
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: Ask a doctor.
-
INACTIVE INGREDIENTS
PHYLLOSTACHYS BAMBUSOIDES JUICE, CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, PHENYL TRIMETHICONE, PEG-10 DIMETHICONE, BUTYLENE GLYCOL, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, ALCOHOL, TITANIUM DIOXIDE (CI 77891), LAURYL PEG-9 POLY[1]DIMETHYLSILOXYETHYL DIMETHICONE, ACRYLATES/ETHYLHEXYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, POLYHYDROXYSTEARIC ACID, IRON OXIDES (CI 77492), SOD[1]IUM CHLORIDE, POLYMETHYL METHACRYLATE, ALUMINUM HYDROXIDE, DISTEARDIMONI[1]UM HECTORITE, FRAGRANCE / PARFUM, STEARIC ACID, PHENOXYETHANOL, TRIETHO[1]XYCAPRYLYLSILANE, ETHYLHEXYL PALMITATE, LECITHIN, ISOSTEARIC ACID, ISOPROPYL PALMITATE, IRON OXIDES (CI 77491), POLYGLYCERYL-3 POLYRICINOLEATE, ACRYLATES/ STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, DIMETHICONE, ETHYL[1]HEXYLGLYCERIN, TRIMETHYLSILOXYSILICATE, DISODIUM EDTA, IRON OXIDES (CI 77499), DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, LINALOOL, WATER / AQUA / EAU, GERANIOL, ALPHA-ISOMETHYL IONONE, LIMONENE, CITRONELLOL, TOCOPHEROL, CAME[1]LLIA SINENSIS LEAF EXTRACT, CAPRYLYL GLYCOL, 1,2-HEXANEDIOL
- OTHER INFORMATION
- Questions?
- PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 104 LIGHT/MEDIUM (PINK)
- PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 106 MEDIUM (PINK)
- PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 204 LIGHT/MEDIUM (YELLOW)
- PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 208 MEDIUM (YELLOW)
-
INGREDIENTS AND APPEARANCE
AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.106
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 15 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.6225 g in 15 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.47 g in 15 g Inactive Ingredients Ingredient Name Strength LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TOCOPHEROL (UNII: R0ZB2556P8) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TRIMETHYLSILOXYSILICATE (M/Q 0.66) (UNII: 5041RX63GN) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHYLLOSTACHYS RETICULATA RESIN (UNII: KX390ME4SP) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) ALCOHOL (UNII: 3K9958V90M) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYL PALMITATE (UNII: 2865993309) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-512-21 2 in 1 CARTON 12/05/2012 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/05/2012 AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.208
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-514 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 15 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.6225 g in 15 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.47 g in 15 g Inactive Ingredients Ingredient Name Strength PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) ALCOHOL (UNII: 3K9958V90M) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYL PALMITATE (UNII: 2865993309) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TOCOPHEROL (UNII: R0ZB2556P8) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TRIMETHYLSILOXYSILICATE (M/Q 0.66) (UNII: 5041RX63GN) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHYLLOSTACHYS RETICULATA RESIN (UNII: KX390ME4SP) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-514-21 2 in 1 CARTON 12/05/2012 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/05/2012 AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.104
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 15 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.6225 g in 15 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.47 g in 15 g Inactive Ingredients Ingredient Name Strength LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TOCOPHEROL (UNII: R0ZB2556P8) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TRIMETHYLSILOXYSILICATE (M/Q 0.66) (UNII: 5041RX63GN) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHYLLOSTACHYS RETICULATA RESIN (UNII: KX390ME4SP) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) ALCOHOL (UNII: 3K9958V90M) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYL PALMITATE (UNII: 2865993309) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-511-21 2 in 1 CARTON 12/05/2012 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/05/2012 AMOREPACIFIC COLOR CONTROL CUSHION COMPACT NO.204
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-513 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 15 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.6225 g in 15 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.47 g in 15 g Inactive Ingredients Ingredient Name Strength LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TOCOPHEROL (UNII: R0ZB2556P8) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TRIMETHYLSILOXYSILICATE (M/Q 0.66) (UNII: 5041RX63GN) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHYLLOSTACHYS RETICULATA RESIN (UNII: KX390ME4SP) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) ALCOHOL (UNII: 3K9958V90M) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYL PALMITATE (UNII: 2865993309) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-513-21 2 in 1 CARTON 12/05/2012 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/05/2012 Labeler - AMOREPACIFIC CORPORATION (631035289)