USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: Ask a doctor.
INACTIVE INGREDIENTS
PHYLLOSTACHYS BAMBUSOIDES JUICE, CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, PHENYL TRIMETHICONE, PEG-10 DIMETHICONE, BUTYLENE GLYCOL, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, ALCOHOL, TITANIUM DIOXIDE (CI 77891), LAURYL PEG-9 POLY[1]DIMETHYLSILOXYETHYL DIMETHICONE, ACRYLATES/ETHYLHEXYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, POLYHYDROXYSTEARIC ACID, IRON OXIDES (CI 77492), SOD[1]IUM CHLORIDE, POLYMETHYL METHACRYLATE, ALUMINUM HYDROXIDE, DISTEARDIMONI[1]UM HECTORITE, FRAGRANCE / PARFUM, STEARIC ACID, PHENOXYETHANOL, TRIETHO[1]XYCAPRYLYLSILANE, ETHYLHEXYL PALMITATE, LECITHIN, ISOSTEARIC ACID, ISOPROPYL PALMITATE, IRON OXIDES (CI 77491), POLYGLYCERYL-3 POLYRICINOLEATE, ACRYLATES/ STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, DIMETHICONE, ETHYL[1]HEXYLGLYCERIN, TRIMETHYLSILOXYSILICATE, DISODIUM EDTA, IRON OXIDES (CI 77499), DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, LINALOOL, WATER / AQUA / EAU, GERANIOL, ALPHA-ISOMETHYL IONONE, LIMONENE, CITRONELLOL, TOCOPHEROL, CAME[1]LLIA SINENSIS LEAF EXTRACT, CAPRYLYL GLYCOL, 1,2-HEXANEDIOL
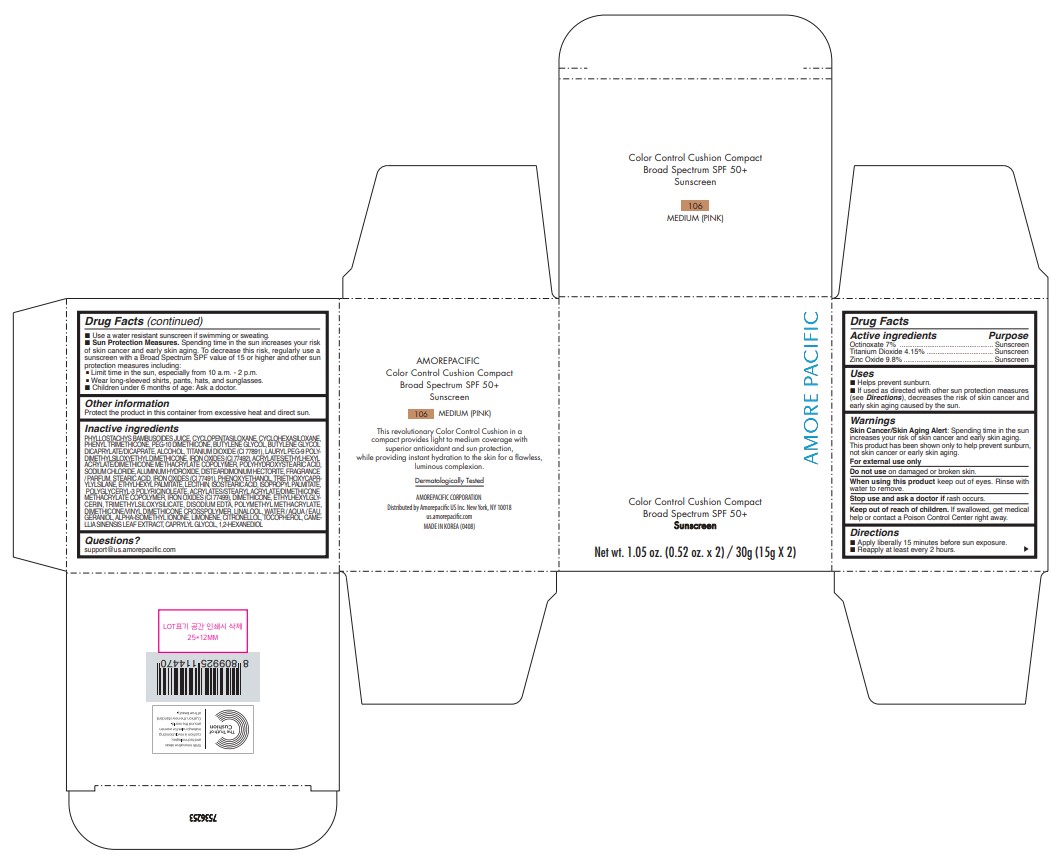
PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 104 LIGHT/MEDIUM (PINK)
Amore Pacific
Color Control Cushion Compact
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)
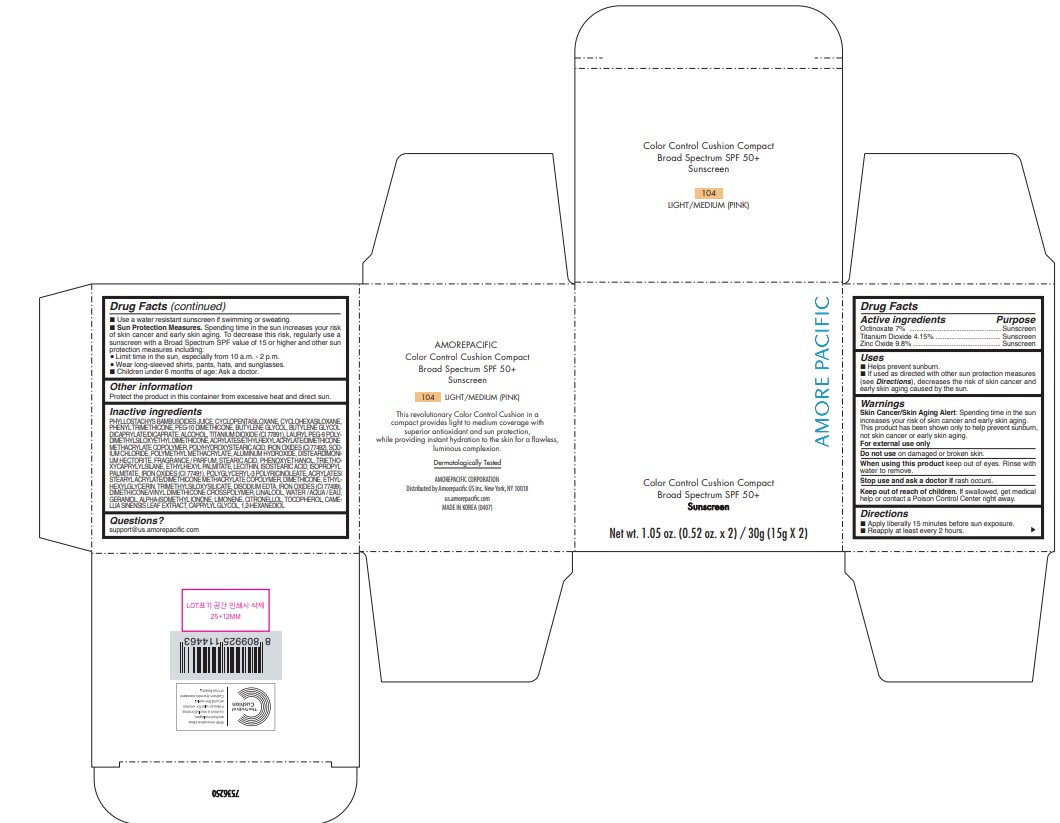
PRINCIPAL DISPLAY PANEL - 30 g Container Carton - 106 MEDIUM (PINK)
AMORE PACIFIC
COLOR CONTROL CUSHION COMPACT
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)