Label: RECURO VAGINAL- povidone iodine douche
-
Contains inactivated NDC Code(s)
NDC Code(s): 51670-100-01 - Packager: IONA PHARMACY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 25, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- DESCRIPTION
- INACTIVE INGREDIENT
-
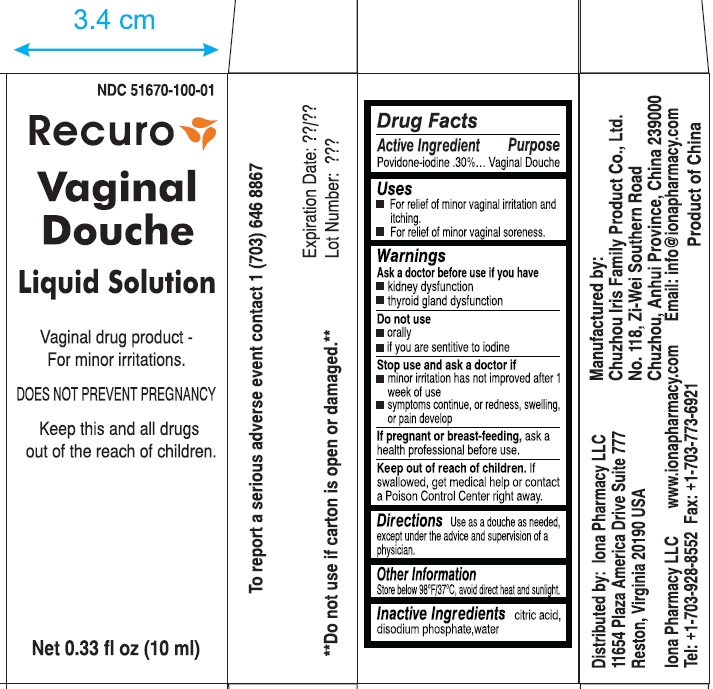
PRINCIPAL DISPLAY PANEL
NDC 51670-100-01
This unit not relabeled for retail sale.
Recuro
Vaginal Douche
Liquid Solution
Vaginal Drug Product- For minor irritations
DOES NOT PREVENT PREGNANCY
keep this and all drugs out of the reach of children.
net 0.33 fl oz (10ml)
**do not use if carton is open or damaged.**
To report a serious adverse event contact 1 (703) 646-8867
Expiration date Lot Number
Distributed by Iona Pharmacy LLC
11654 Plaza America Drive Suite 777
Reston, Virginia 20190 USA
Iona Pharmacy LLC
www.iona pharmacy .com
Tel: +1-703-928-8552 Fax: 1-703-773-6921
Manufactured by:
Chuzhou Iris Family Product Co., Ltd.
No. 118, Ze-Wei Southern Road
Chuzhou, Anhui Province, China 239000
Email: info@ionapharmacy.com
Product of China
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RECURO VAGINAL
povidone iodine doucheProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51670-100 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) POVIDONE-IODINE 0.30 mL in 100 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHOSPHATE ION (UNII: NK08V8K8HR) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51670-100-01 1 in 1 BOX 1 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 10/25/2010 Labeler - IONA PHARMACY (962591116)