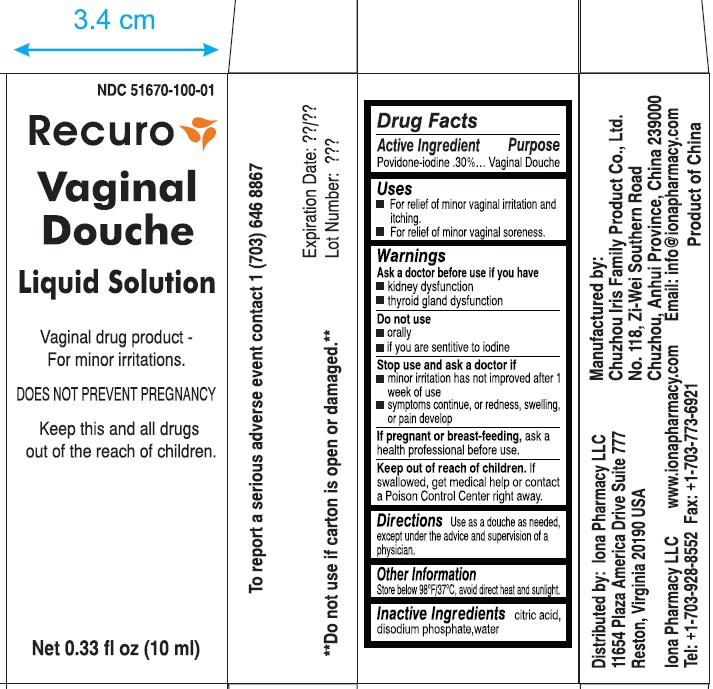
Stop use and ask a doctor if
minor irritation has not improved after 1 week of use
symptoms continue or redness, swelling or pain develop
Keep out of reach of children. If swallowed, get medical help or contact a Poison control center right away.
NDC 51670-100-01
This unit not relabeled for retail sale.
Recuro
Vaginal Douche
Liquid Solution
Vaginal Drug Product- For minor irritations
DOES NOT PREVENT PREGNANCY
keep this and all drugs out of the reach of children.
net 0.33 fl oz (10ml)
**do not use if carton is open or damaged.**
To report a serious adverse event contact 1 (703) 646-8867
Expiration date Lot Number
Distributed by Iona Pharmacy LLC
11654 Plaza America Drive Suite 777
Reston, Virginia 20190 USA
Iona Pharmacy LLC
www.iona pharmacy .com
Tel: +1-703-928-8552 Fax: 1-703-773-6921
Manufactured by:
Chuzhou Iris Family Product Co., Ltd.
No. 118, Ze-Wei Southern Road
Chuzhou, Anhui Province, China 239000
Email: info@ionapharmacy.com
Product of China