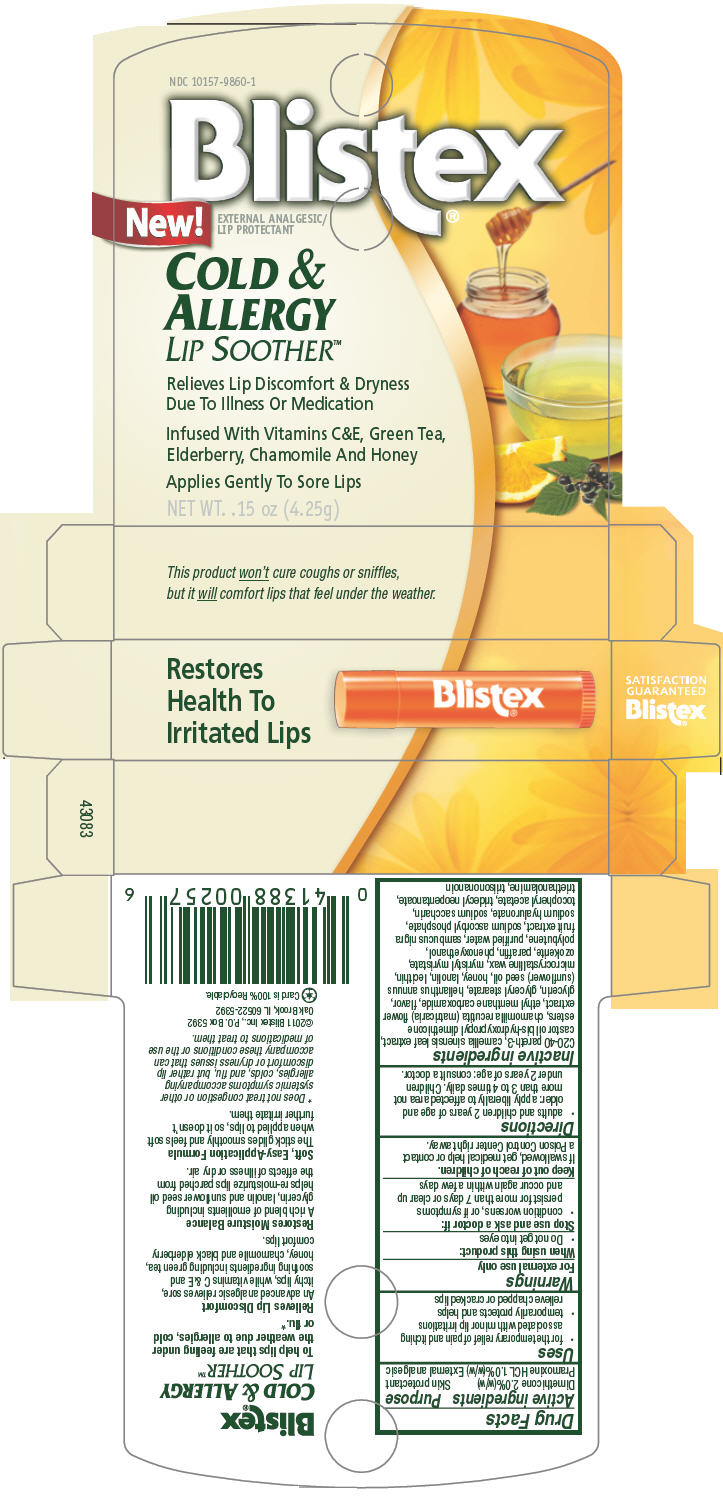
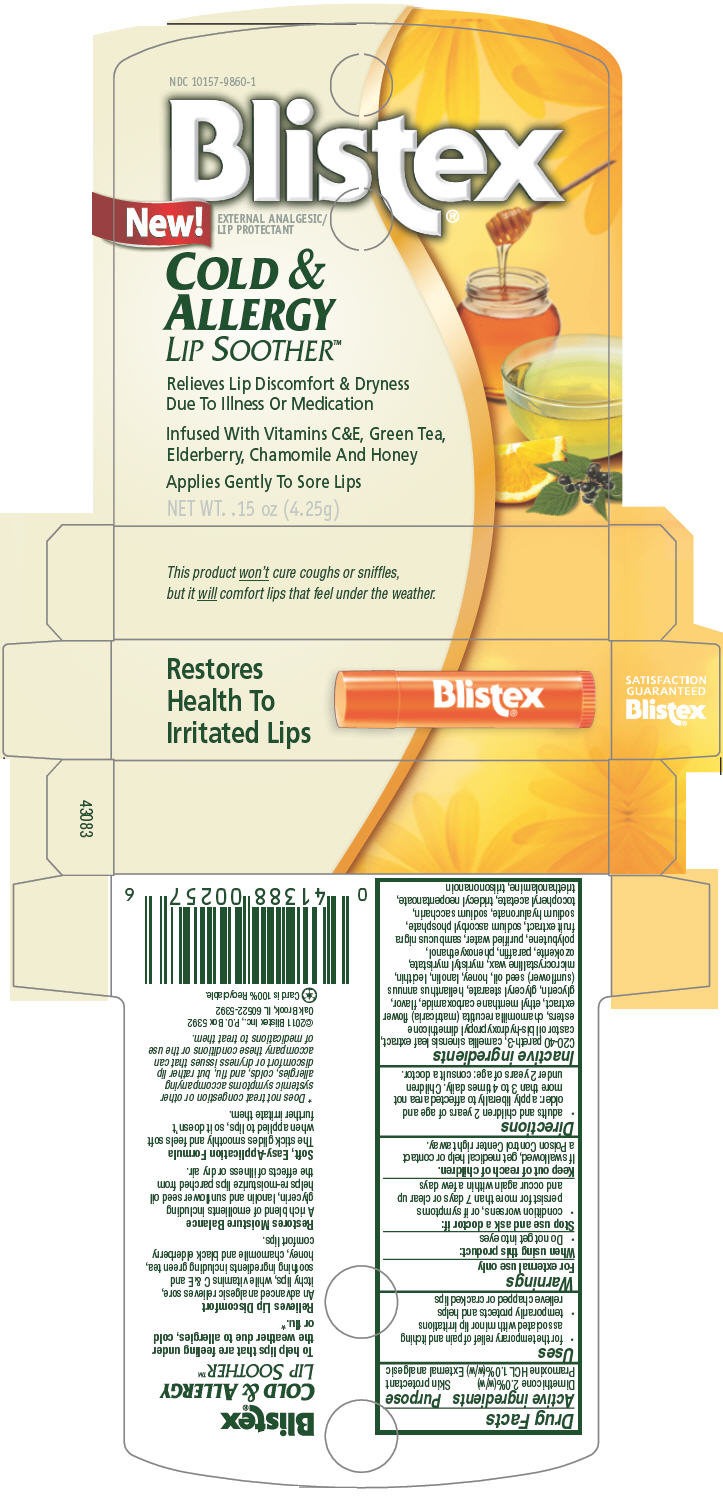
Label: BLISTEX COLD AND ALLERGY LIP SOOTHER- dimethicone and pramoxine hydrochloride stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 10157-9860-1 - Packager: Blistex Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 12, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
C20-40 pareth-3, camellia sinensis leaf extract, castor oil bis-hydroxypropyl dimethicone esters, chamomilla recutita (matricaria) flower extract, ethyl menthane carboxamide, flavor, glycerin, glyceryl stearate, helianthus annuus (sunflower) seed oil, honey, lanolin, lecithin, microcrystalline wax, myristyl myristate, ozokerite, paraffin, phenoxyethanol, polybutene, purified water, sambucus nigra fruit extract, sodium ascorbyl phosphate, sodium hyaluronate, sodium saccharin, tocopheryl acetate, tridecyl neopentanoate, triethanolamine, triisononanoin
- PRINCIPAL DISPLAY PANEL 4.25g Carton
-
INGREDIENTS AND APPEARANCE
BLISTEX COLD AND ALLERGY LIP SOOTHER
dimethicone and pramoxine hydrochloride stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10157-9860 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 2 g in 100 g Pramoxine Hydrochloride (UNII: 88AYB867L5) (Pramoxine - UNII:068X84E056) Pramoxine Hydrochloride 1 g in 100 g Inactive Ingredients Ingredient Name Strength green tea leaf (UNII: W2ZU1RY8B0) chamomile (UNII: FGL3685T2X) ethyl menthane carboxamide (UNII: 6S7S02945H) glycerin (UNII: PDC6A3C0OX) glyceryl monostearate (UNII: 230OU9XXE4) sunflower oil (UNII: 3W1JG795YI) honey (UNII: Y9H1V576FH) lanolin (UNII: 7EV65EAW6H) microcrystalline wax (UNII: XOF597Q3KY) myristyl myristate (UNII: 4042ZC00DY) paraffin (UNII: I9O0E3H2ZE) phenoxyethanol (UNII: HIE492ZZ3T) water (UNII: 059QF0KO0R) european elderberry (UNII: BQY1UBX046) sodium ascorbyl phosphate (UNII: 836SJG51DR) hyaluronate sodium (UNII: YSE9PPT4TH) saccharin sodium (UNII: SB8ZUX40TY) tridecyl neopentanoate (UNII: 3Z8H1DA7J5) tangerine (UNII: KH3E3096OO) ceresin (UNII: Q1LS2UJO3A) trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10157-9860-1 1 in 1 CARTON 1 4.25 g in 1 CYLINDER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 07/01/2011 Labeler - Blistex Inc (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc 005126354 MANUFACTURE