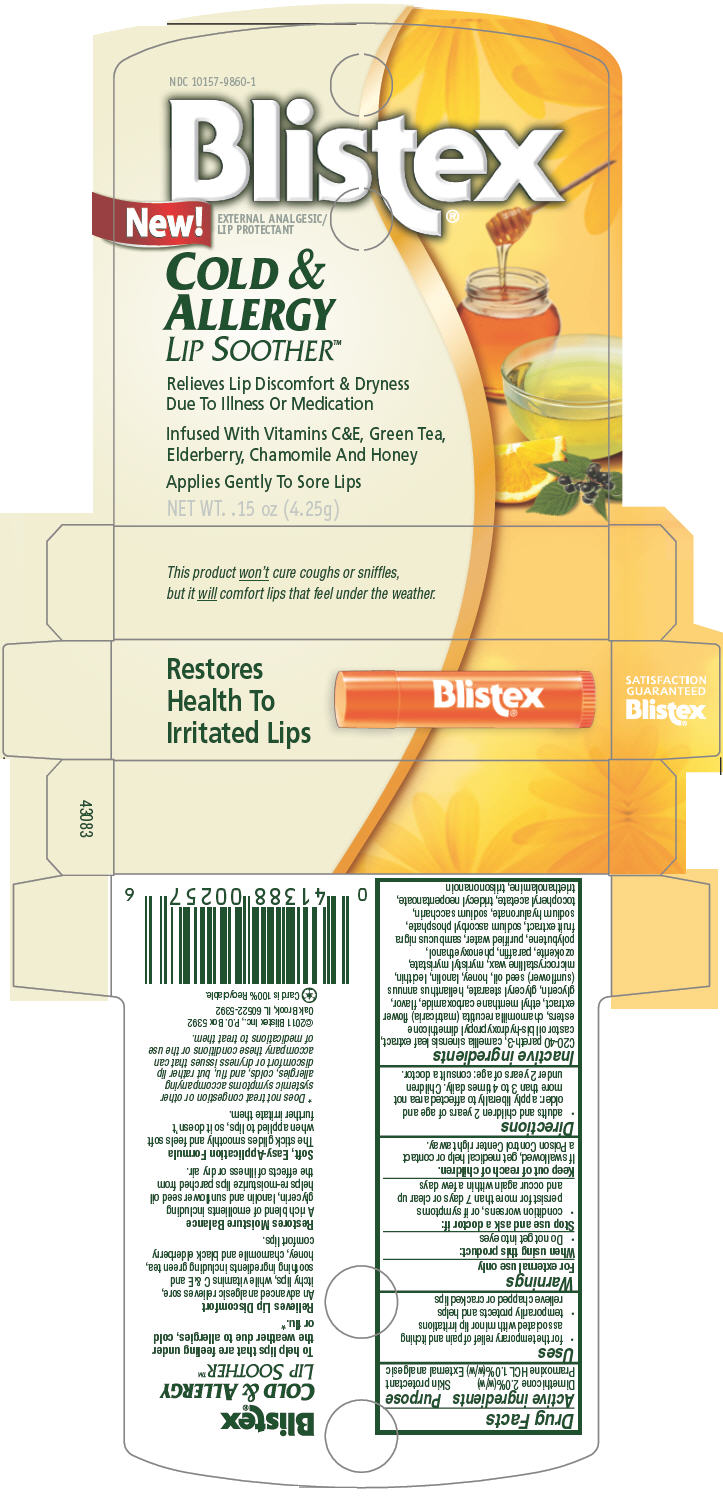
Uses
- for the temporary relief of pain and itching associated with minor lip irritations
- temporarily protects and helps relieve chapped or cracked lips
Warnings
For external use only
Directions
- adults and children 2 years of age and older: apply liberally to affected area not more than 3 to 4 times daily. Children under 2 years of age: consult a doctor.
Inactive ingredients
C20-40 pareth-3, camellia sinensis leaf extract, castor oil bis-hydroxypropyl dimethicone esters, chamomilla recutita (matricaria) flower extract, ethyl menthane carboxamide, flavor, glycerin, glyceryl stearate, helianthus annuus (sunflower) seed oil, honey, lanolin, lecithin, microcrystalline wax, myristyl myristate, ozokerite, paraffin, phenoxyethanol, polybutene, purified water, sambucus nigra fruit extract, sodium ascorbyl phosphate, sodium hyaluronate, sodium saccharin, tocopheryl acetate, tridecyl neopentanoate, triethanolamine, triisononanoin
PRINCIPAL DISPLAY PANEL 4.25g Carton
NDC 10157-9860-1
Blistex®
New!
EXTERNAL ANALGESIC/
LIP PROTECTANT
COLD &
ALLERGY
LIP SOOTHER™
Relieves Lip Discomfort & Dryness
Due To Illness Or Medication
Infused With Vitamins C&E, Green Tea,
Elderberry, Chamomile And Honey
Applies Gently To Sore Lips
NET WT. .15 oz (4.25g)