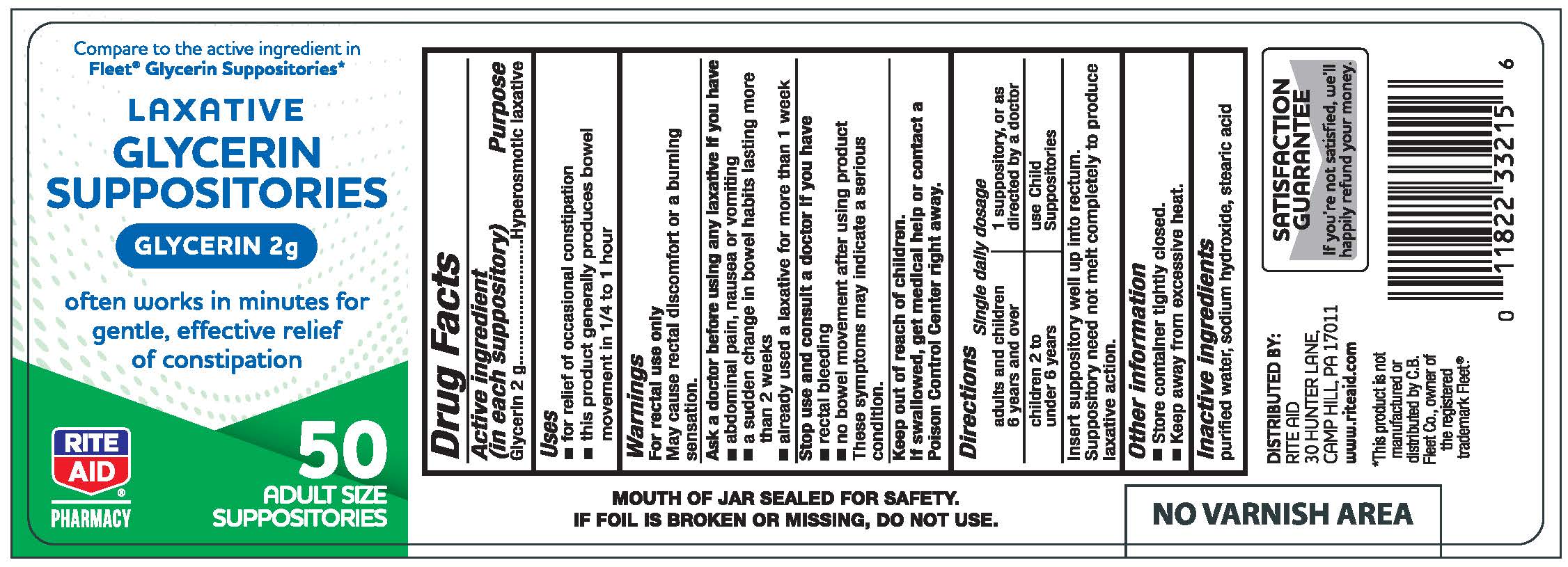
Label: GLYCERIN- glycerin suppository
- NDC Code(s): 11822-3312-5, 11822-3312-9
- Packager: Rite Aid Corporation
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each suppository)
- Purpose
- Uses
-
Warnings
For rectal use only
May cause rectal discomfort of burning sensation
Ask a Doctor before using any laxative if you have
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
- Directions
- Other information
- Inactive ingredients
- Glycerin Suppositories, 50 count
-
INGREDIENTS AND APPEARANCE
GLYCERIN
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-3312 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-3312-9 100 in 1 JAR; Type 0: Not a Combination Product 10/14/2011 2 NDC:11822-3312-5 50 in 1 JAR; Type 0: Not a Combination Product 10/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/14/2011 Labeler - Rite Aid Corporation (014578892) Registrant - DSC Laboratories Inc. (097807374) Establishment Name Address ID/FEI Business Operations DSC Laboratories, Inc. 097807374 manufacture(11822-3312)