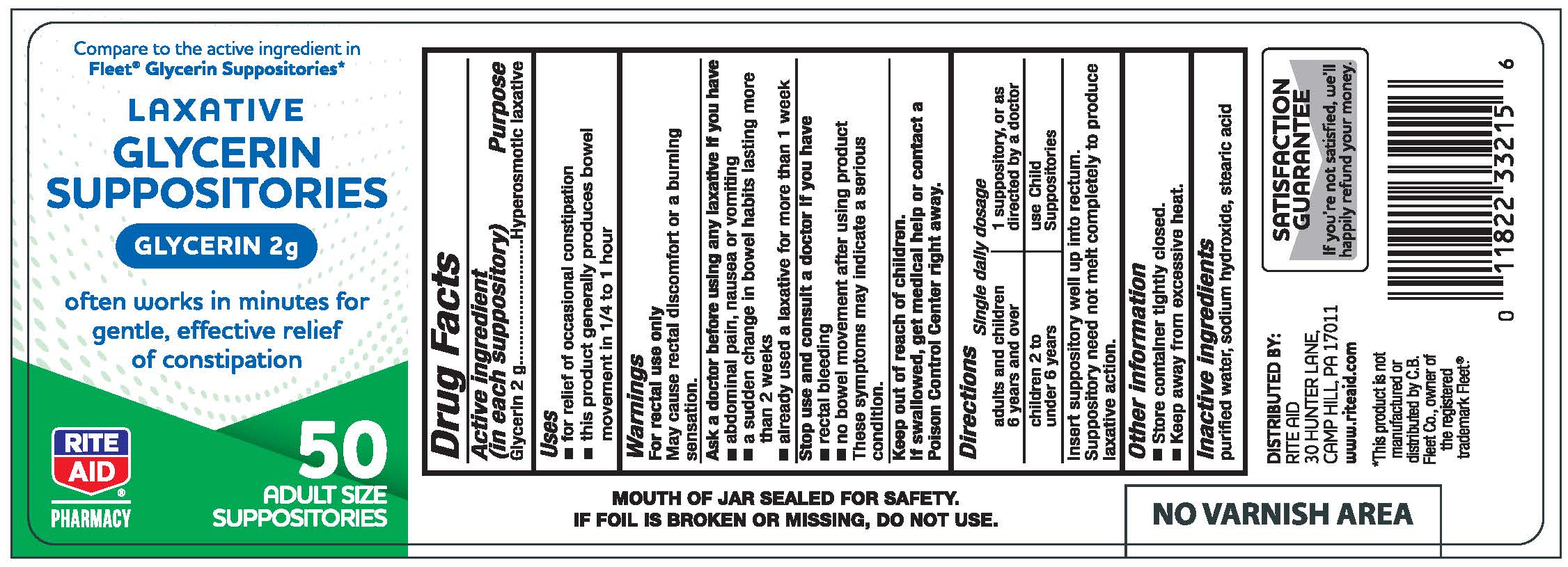
Active ingredient (in each suppository)
Glycerin 2 g
Purpose
Hyperosmotic laxative
Uses
- for relief of occasional constipation
- this product generally produces a bowel movement in 1/4 to 1 hour
Warnings
For rectal use only
May cause rectal discomfort of burning sensation
Ask a Doctor before using any laxative if you have
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
Stop use and consult a doctor if you have
- rectal bleeding
- no bowel movement after using this product
These symptoms may indicate a serious condition.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Single daily dosage
adults and children 6 years and over 1 suppository, or as directed by a doctor
children 2 to under 6 years use Child Suppositories
Insert suppository well up into rectum.
Suppository need not melt completely to produce laxative action.
Other information
- Store container tightly closed.
- Keep away from excessive heat.
Inactive ingredients
purified water, sodium hydroxide, stearic acid
Glycerin Suppositories, 50 count
The product package shown above represents a sample of that currently in use. Additional packaging may also be available.
RAGlycerinSupp50ct
Distributed by
Rite Aid
30 Hunter Lane
Camp Hill, PA 17011
