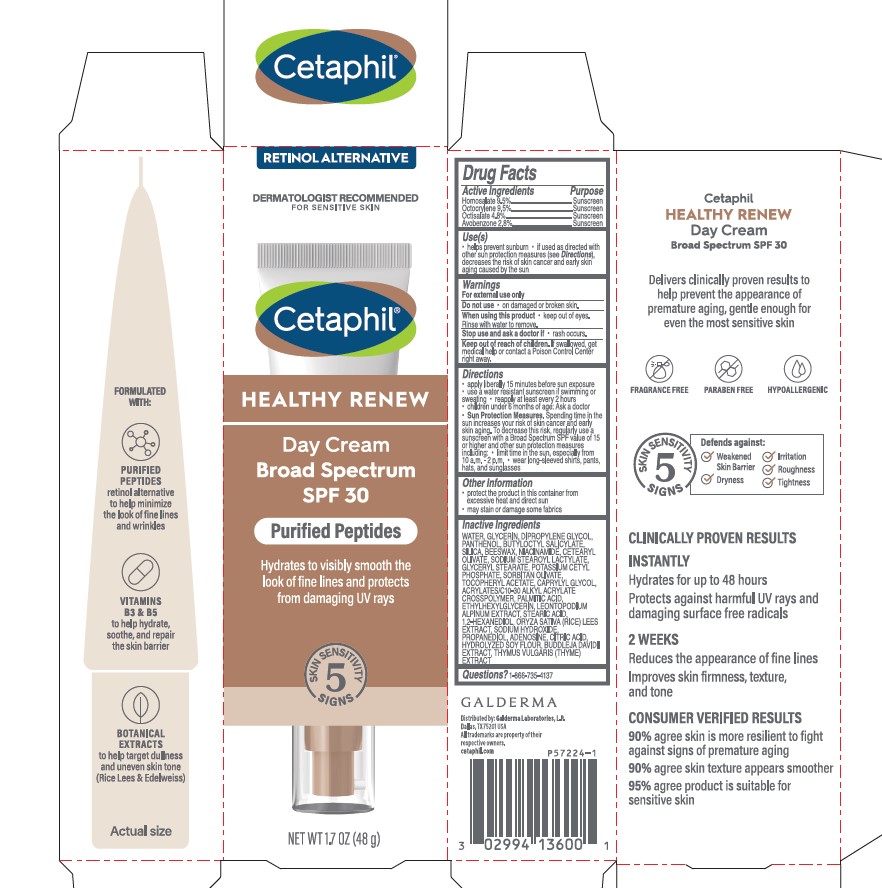
Label: CETAPHIL HEALTHY RENEW DAY CREAM BROAD SPECTRUM SPF 30- homosalate, octocrylene, octisalate, avobenzone lotion
- NDC Code(s): 0299-4136-00
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients.....Purpose
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients
WATER, GLYCERIN, DIPROPYLENE GLYCOL, PANTHENOL, BUTYLOCTYL SALICYLATE, SILICA, BEESWAX, NIACINAMIDE, CETEARYL OLIVATE, SODIUM STEAROYL LACTYLATE, GLYCERYL STEARATE, POTASSIUM CETYL PHOSPHATE, SORBITAN OLIVATE, TOCOPHERYL ACETATE, CAPRYLYL GLYCOL, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, PALMITIC ACID, ETHYLHEXYLGLYCERIN, LEONTOPODIUM ALPINUM EXTRACT, STEARIC ACID, 1,2-HEXANEDIOL, ORYZA SATIVA (RICE) LEES EXTRACT, SODIUM HYDROXIDE, PROPANEDIOL, ADENOSINE, CITRIC ACID, HYDROLYZED SOY FLOUR, BUDDLEJA DAVIDII EXTRACT, THYMUS VULGARIS (THYME) EXTRACT
- Questions or comments?
-
Principal Display Panel - 1.7 OZ Carton
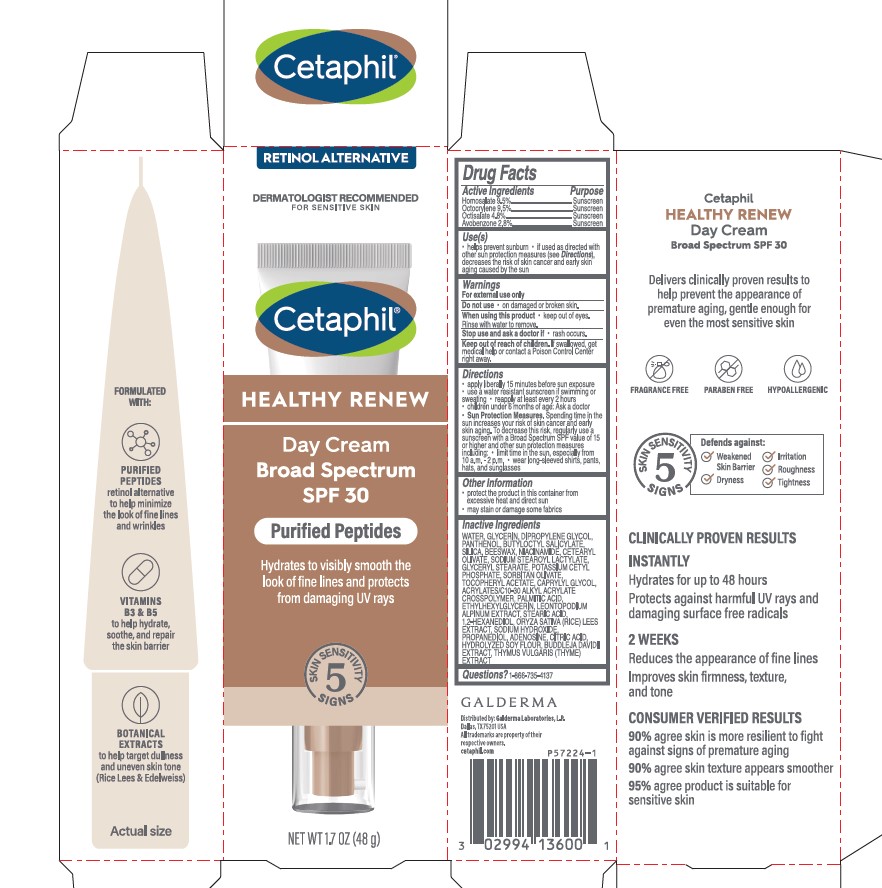
RETINOL ALTERNATIVE
Dermatologist Recommended
For Sensitive Skin
Cetaphil®
HEALTHY RENEWDay Cream
Broad Spectrum
SPF 30
Purified Peptides
Hydrates to visibly smooth the
look of fine lines and protects
from damaging UV rays
Five Skin Sensitivity Signs
NET WT 1.7 OZ (48 g)
Distributed by:
Galderma Laboratories, L.P., Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com
P57224-1
-
INGREDIENTS AND APPEARANCE
CETAPHIL HEALTHY RENEW DAY CREAM BROAD SPECTRUM SPF 30
homosalate, octocrylene, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4136 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 95 mg in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 95 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 48 mg in 1 g Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 28 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Dipropylene Glycol (UNII: E107L85C40) Panthenol (UNII: WV9CM0O67Z) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Silicon Dioxide (UNII: ETJ7Z6XBU4) Yellow Wax (UNII: 2ZA36H0S2V) Niacinamide (UNII: 25X51I8RD4) Cetearyl Olivate (UNII: 58B69Q84JO) Sodium Stearoyl Lactylate (UNII: IN99IT31LN) Glyceryl Monostearate (UNII: 230OU9XXE4) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) Sorbitan Olivate (UNII: MDL271E3GR) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Caprylyl Glycol (UNII: 00YIU5438U) Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Palmitic Acid (UNII: 2V16EO95H1) Ethylhexylglycerin (UNII: 147D247K3P) Leontopodium Nivale Subsp. Alpinum Root (UNII: SDW6SS1K6I) Stearic Acid (UNII: 4ELV7Z65AP) 1,2-Hexanediol (UNII: TR046Y3K1G) Rice Germ (UNII: 7N2B70SFEZ) Sodium Hydroxide (UNII: 55X04QC32I) Propanediol (UNII: 5965N8W85T) Adenosine (UNII: K72T3FS567) Citric Acid Monohydrate (UNII: 2968PHW8QP) Hydrolyzed Soy Protein (Enzymatic; 2000 Mw) (UNII: 1394NXB9L6) Buddleja Davidii Whole (UNII: 8JZ8B2V1OV) Thyme (UNII: CW657OBU4N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4136-00 1 in 1 CARTON 04/12/2023 1 48 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/12/2023 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations ENGLEWOOD LAB, INC. 172198223 manufacture(0299-4136)