Active Ingredients.....Purpose
Homosalate 9.5% .................Sunscreen
Octocrylene 9.5% .........… Sunscreen
Octisalate 4.8% ...............… Sunscreen
Avobenzone 2.8% ................. Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
WATER, GLYCERIN, DIPROPYLENE GLYCOL, PANTHENOL, BUTYLOCTYL SALICYLATE, SILICA, BEESWAX, NIACINAMIDE, CETEARYL OLIVATE, SODIUM STEAROYL LACTYLATE, GLYCERYL STEARATE, POTASSIUM CETYL PHOSPHATE, SORBITAN OLIVATE, TOCOPHERYL ACETATE, CAPRYLYL GLYCOL, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, PALMITIC ACID, ETHYLHEXYLGLYCERIN, LEONTOPODIUM ALPINUM EXTRACT, STEARIC ACID, 1,2-HEXANEDIOL, ORYZA SATIVA (RICE) LEES EXTRACT, SODIUM HYDROXIDE, PROPANEDIOL, ADENOSINE, CITRIC ACID, HYDROLYZED SOY FLOUR, BUDDLEJA DAVIDII EXTRACT, THYMUS VULGARIS (THYME) EXTRACT
Principal Display Panel - 1.7 OZ Carton
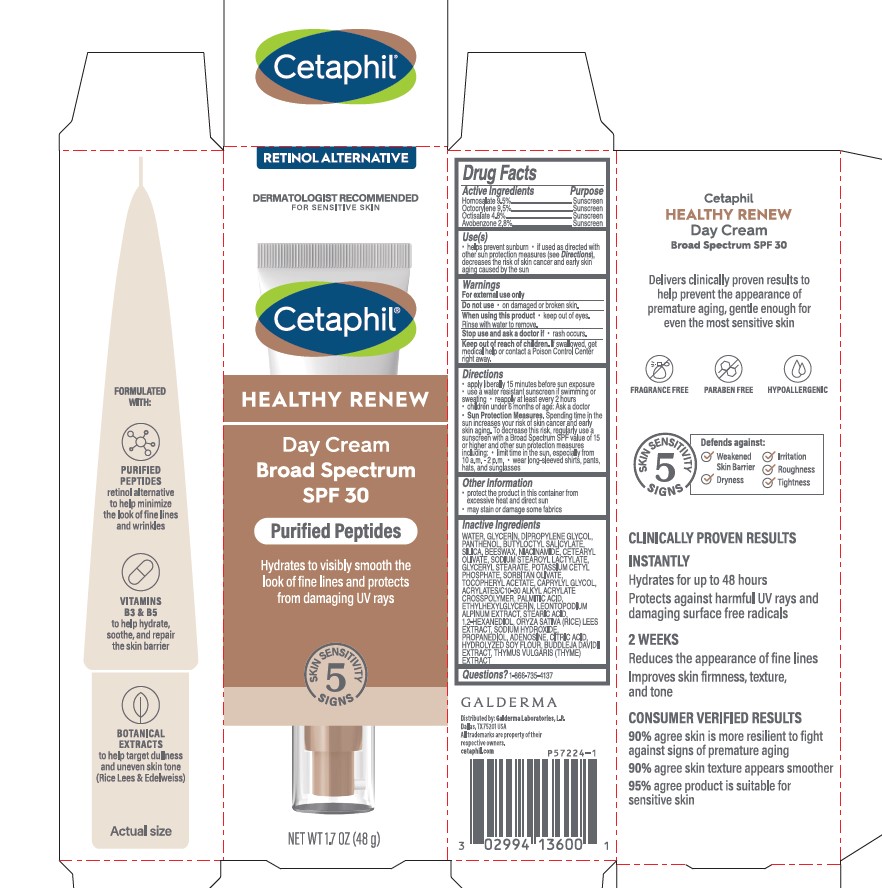
RETINOL ALTERNATIVE
Dermatologist Recommended
For Sensitive Skin
Cetaphil®
HEALTHY RENEW
Day Cream
Broad Spectrum
SPF 30
Purified Peptides
Hydrates to visibly smooth the
look of fine lines and protects
from damaging UV rays
Five Skin Sensitivity Signs
NET WT 1.7 OZ (48 g)
Distributed by:
Galderma Laboratories, L.P., Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com
P57224-1