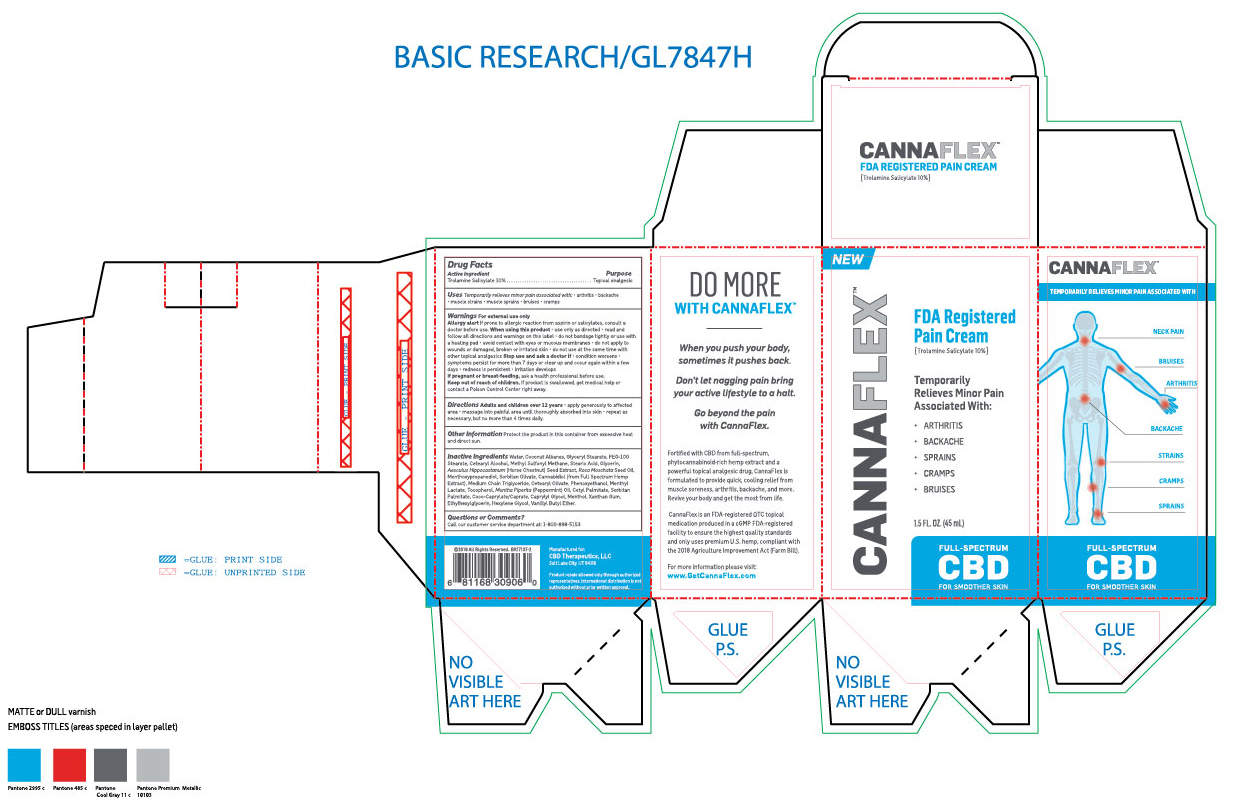
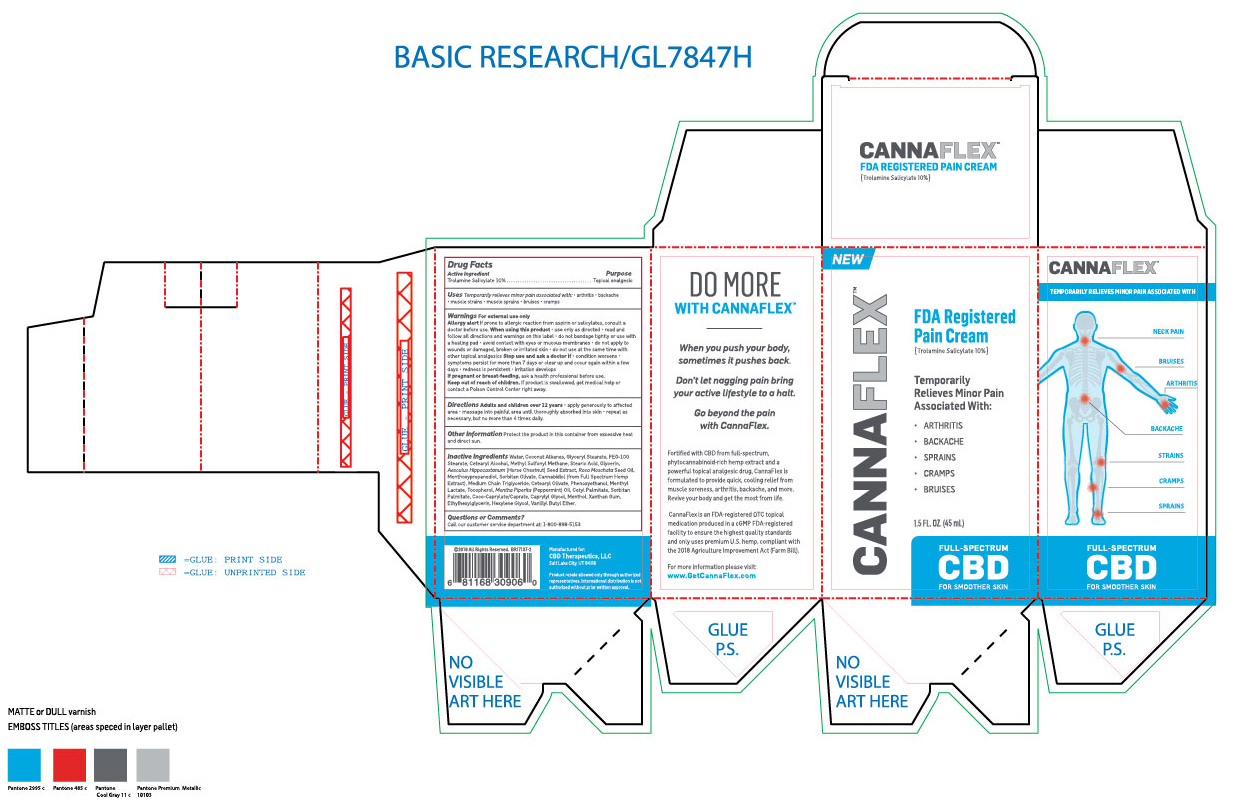
Label: CANNAFLEX- trolamine salicylate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 62742-4177-1, 62742-4177-2 - Packager: ALLURE LABS INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 16, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
-
WHEN USING
When using this product: Use only as directed. Read and follow all directions and warnings on this label. Do not bandage tightly or use with a heated pad. Avoid contact with eyes or mucous membranes. Do not apply to wounds or damaged, broken or irritated skin. Do not use at the same time with other topical analgesics.
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Inactive ingredients: Water, Coconut Alkanes, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Methyl Sulfonyl Methane, Stearic Acid, Glycerin, Aesculus Hippocastannum (Horse Chestnut) Seed Extract, Rosa Moschata Seed Oil, Menthoxypropanediol, Sorbitan Olivate, Cannabidiol (From full spectrum hemp extract), Medium Chain Triglyceride, Cetearyl Olivate, Phenoxyethanol, Menthyl Lactate, Tocopherol, Mentha Piperita (Pepperment) Oil, Cetyl Palmitate, Sorbitan Palmitate, Coco-Caprylate/Caprate, Caprylyl Glycol, Menthol, Xanthan Gum, Ethylhexylglycerin, Hexylene Glycol, Vanillyl Butyl Ether.
- DOSAGE & ADMINISTRATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CANNAFLEX
trolamine salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4177 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROLAMINE SALICYLATE (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) TROLAMINE SALICYLATE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) HORSE CHESTNUT (UNII: 3C18L6RJAZ) ROSA MOSCHATA SEED OIL (UNII: T031ZE559T) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) SORBITAN OLIVATE (UNII: MDL271E3GR) CANNABIDIOL (UNII: 19GBJ60SN5) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETEARYL OLIVATE (UNII: 58B69Q84JO) PHENOXYETHANOL (UNII: HIE492ZZ3T) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) TOCOPHEROL (UNII: R0ZB2556P8) PEPPERMINT OIL (UNII: AV092KU4JH) CETYL PALMITATE (UNII: 5ZA2S6B08X) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MENTHOL (UNII: L7T10EIP3A) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) VANILLYL BUTYL ETHER (UNII: S2ULN37C9R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4177-2 1 in 1 CARTON 10/16/2019 1 NDC:62742-4177-1 45 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/16/2019 Labeler - ALLURE LABS INC. (926831603) Registrant - ALLURE LABS INC. (926831603) Establishment Name Address ID/FEI Business Operations ALLURE LABS INC. 926831603 manufacture(62742-4177)