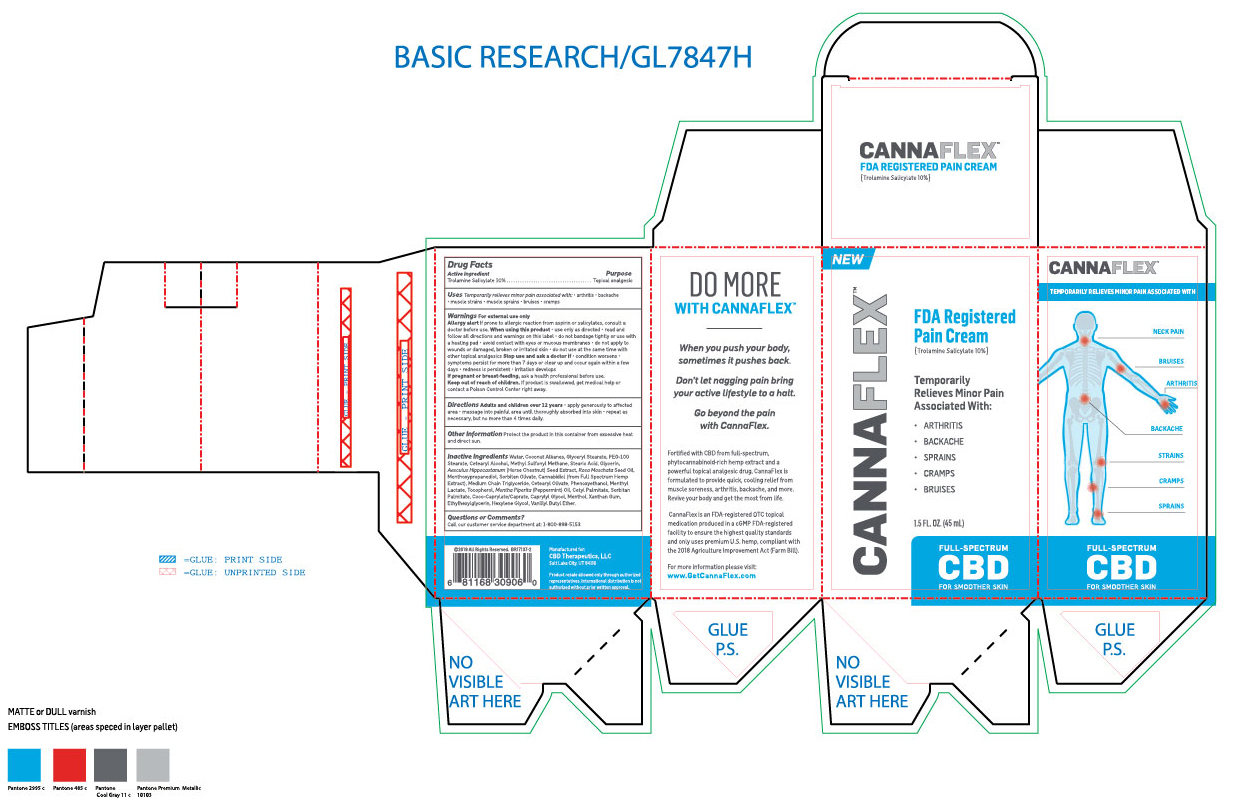
Uses: Temporarily relieves minor pain associated with arthritis, backache, muscle strains, muscle sprains, bruises, cramps.
Allergy alert if prone to allergic reaction from aspirin or salicylates, consult a Doctor before use.
When using this product: Use only as directed. Read and follow all directions and warnings on this label. Do not bandage tightly or use with a heated pad. Avoid contact with eyes or mucous membranes. Do not apply to wounds or damaged, broken or irritated skin. Do not use at the same time with other topical analgesics.
Stop use and ask a doctor if condition worsens. Symptoms persist for more than 7 days or clear up and occur again within a few days. Redness is persistent. Irritation develops.
Keep out of reach of children. If product swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients: Water, Coconut Alkanes, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Methyl Sulfonyl Methane, Stearic Acid, Glycerin, Aesculus Hippocastannum (Horse Chestnut) Seed Extract, Rosa Moschata Seed Oil, Menthoxypropanediol, Sorbitan Olivate, Cannabidiol (From full spectrum hemp extract), Medium Chain Triglyceride, Cetearyl Olivate, Phenoxyethanol, Menthyl Lactate, Tocopherol, Mentha Piperita (Pepperment) Oil, Cetyl Palmitate, Sorbitan Palmitate, Coco-Caprylate/Caprate, Caprylyl Glycol, Menthol, Xanthan Gum, Ethylhexylglycerin, Hexylene Glycol, Vanillyl Butyl Ether.