Label: ATAZANAVIR capsule
-
NDC Code(s):
70771-1591-1,
70771-1591-6,
70771-1592-1,
70771-1592-6, view more70771-1593-1, 70771-1593-3
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 13, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
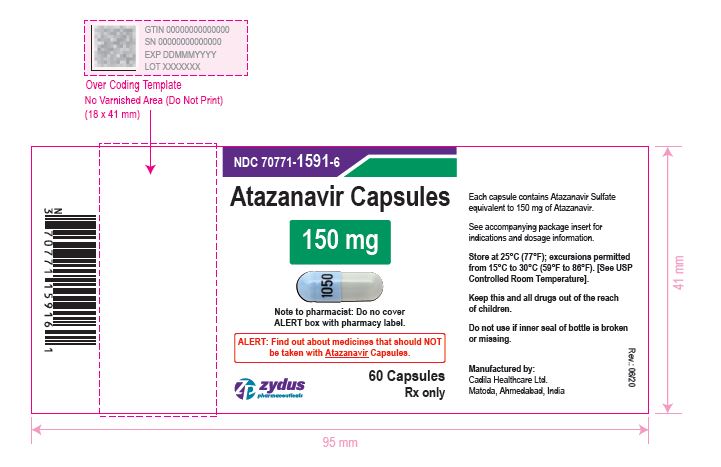
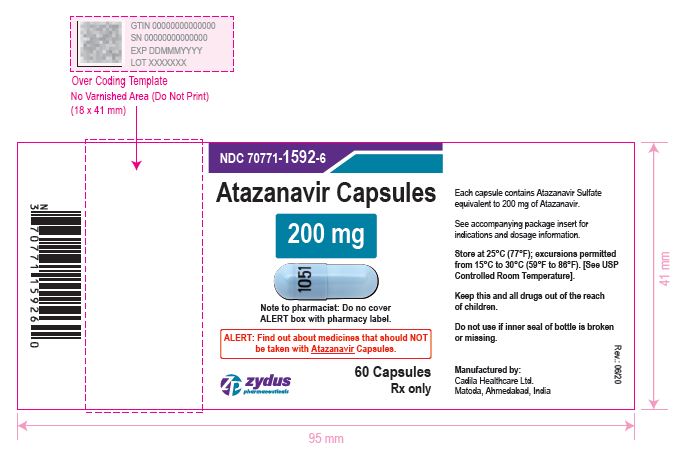
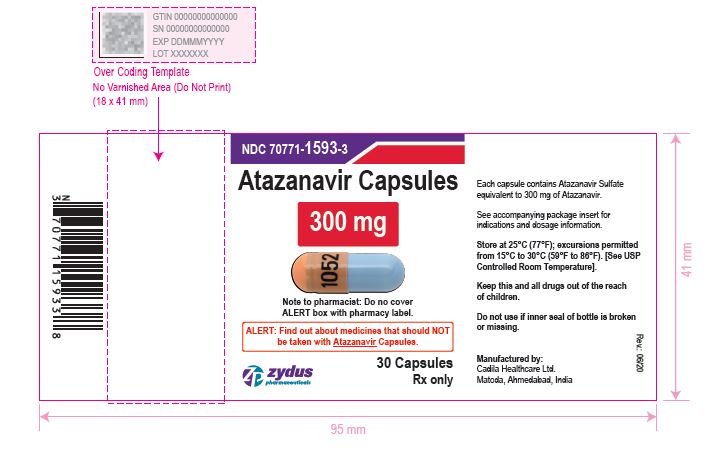
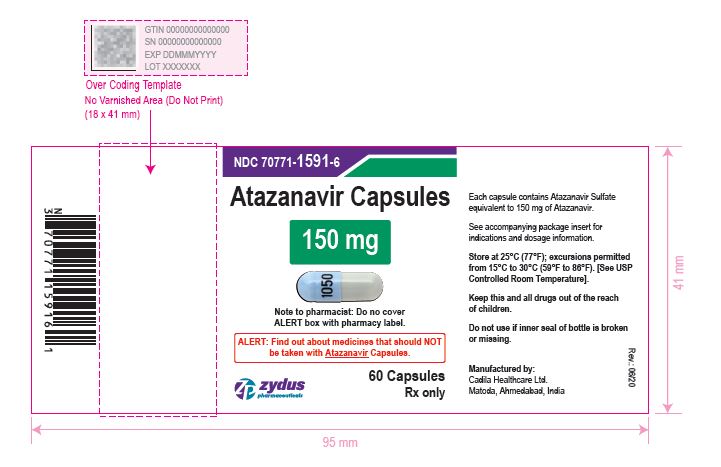
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ATAZANAVIR
atazanavir capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1591 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATAZANAVIR SULFATE (UNII: 4MT4VIE29P) (ATAZANAVIR - UNII:QZU4H47A3S) ATAZANAVIR 150 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 2S7830E561) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color BLUE (opaque blue) , GRAY (grey opaque) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 1050 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1591-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 2 NDC:70771-1591-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210575 05/04/2021 ATAZANAVIR
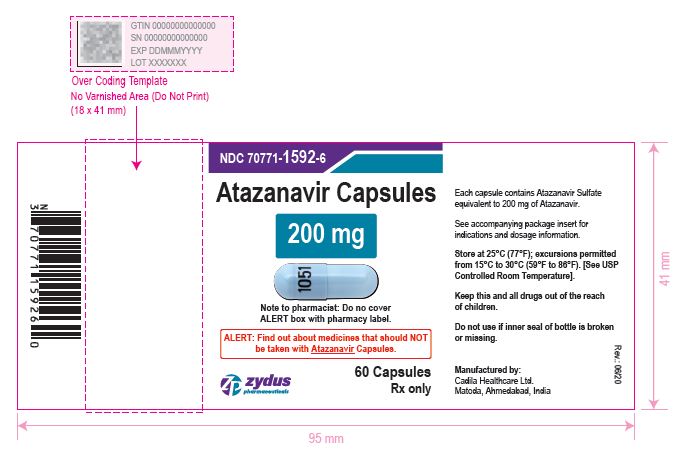
atazanavir capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1592 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATAZANAVIR SULFATE (UNII: 4MT4VIE29P) (ATAZANAVIR - UNII:QZU4H47A3S) ATAZANAVIR 200 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 2S7830E561) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 28 (UNII: 767IP0Y5NH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color BLUE (opaque blue) , BLUE (blue opaque) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code 1051 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1592-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 2 NDC:70771-1592-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210575 05/04/2021 ATAZANAVIR
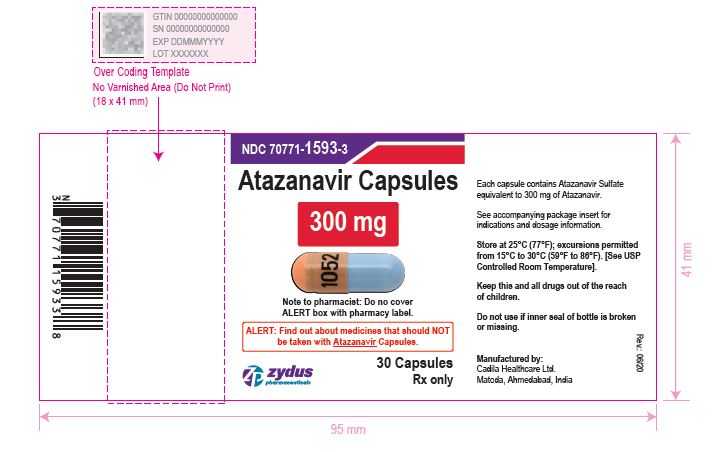
atazanavir capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1593 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATAZANAVIR SULFATE (UNII: 4MT4VIE29P) (ATAZANAVIR - UNII:QZU4H47A3S) ATAZANAVIR 300 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 2S7830E561) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 28 (UNII: 767IP0Y5NH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color ORANGE (orange opaque) , BLUE (blue opaque) Score no score Shape CAPSULE Size 24mm Flavor Imprint Code 1052 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1593-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 2 NDC:70771-1593-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210575 05/04/2021 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (863362789) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 863362789 ANALYSIS(70771-1591, 70771-1592, 70771-1593) , MANUFACTURE(70771-1591, 70771-1592, 70771-1593)