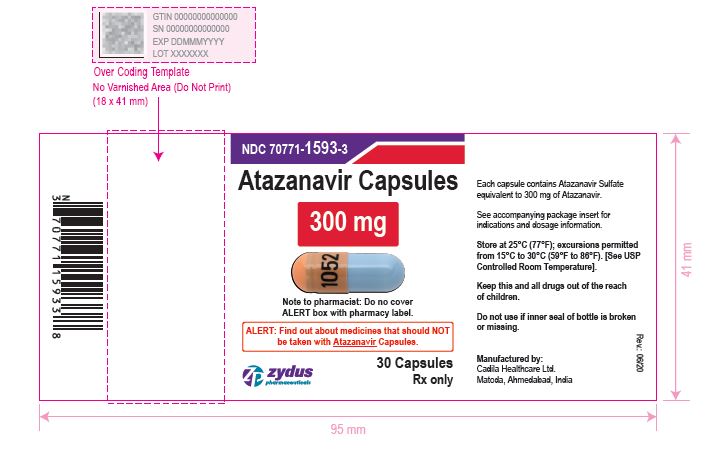
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1591-6 in bottle of 60 Capsules
Atazanavir Capsules, 150 mg
Rx only
60 Capsules
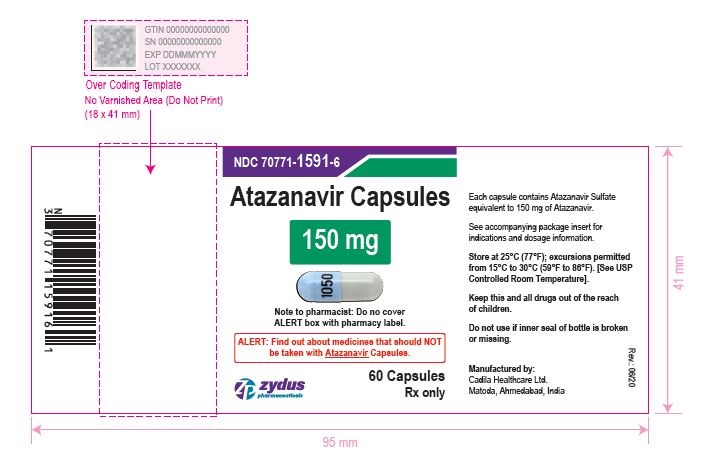
NDC 70771-1592-6 in bottle of 60 Capsules
Atazanavir Capsules, 200 mg
Rx only
60 Capsules
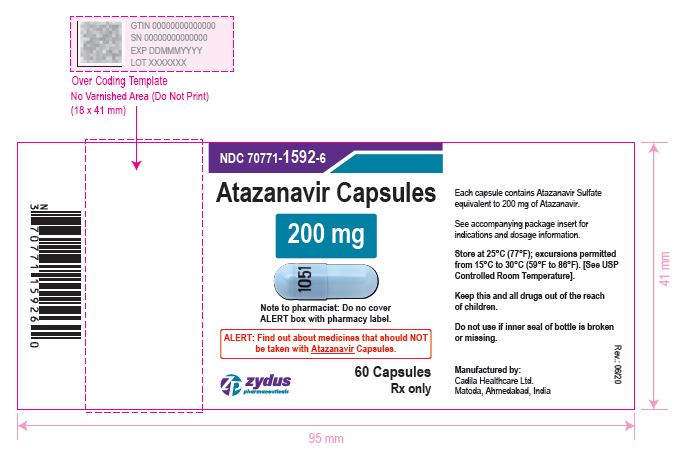
NDC 70771-1593-3 in bottle of 30 Capsules
Atazanavir Capsules, 300 mg
Rx only
30 Capsules