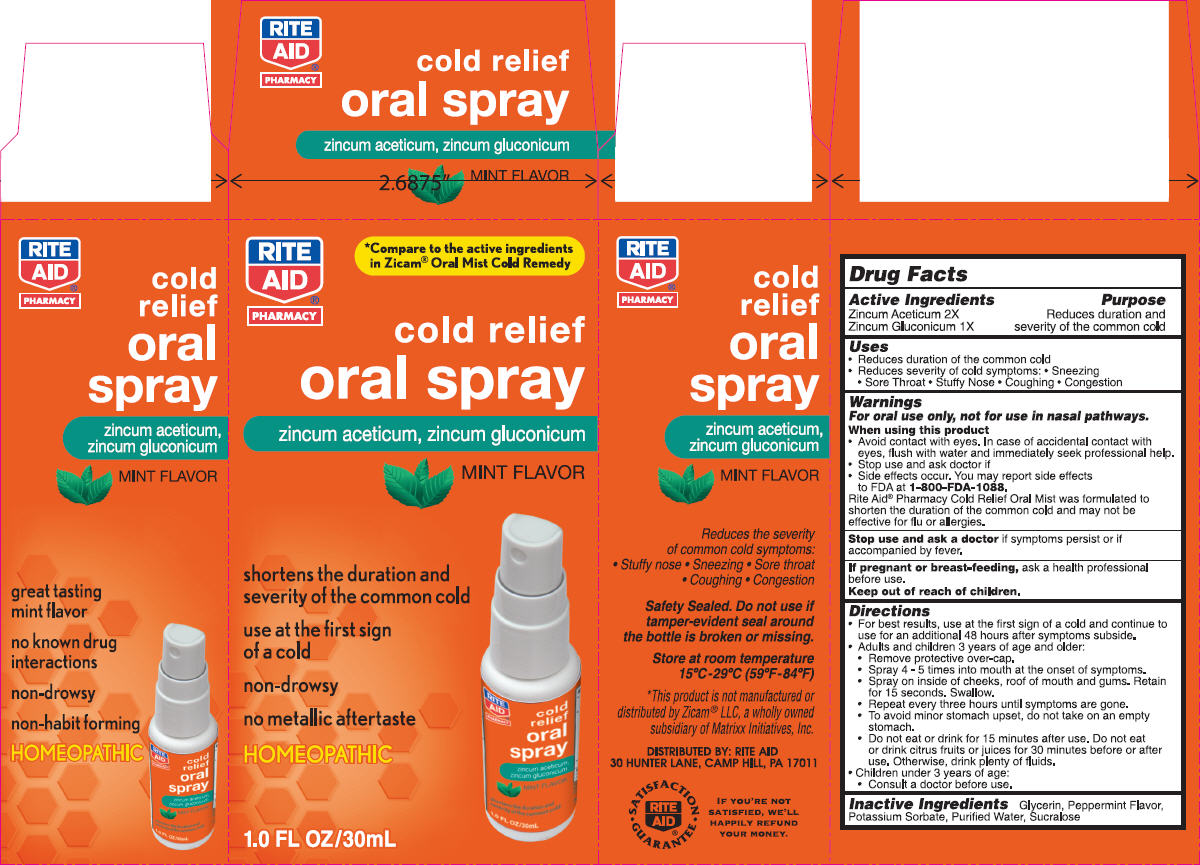
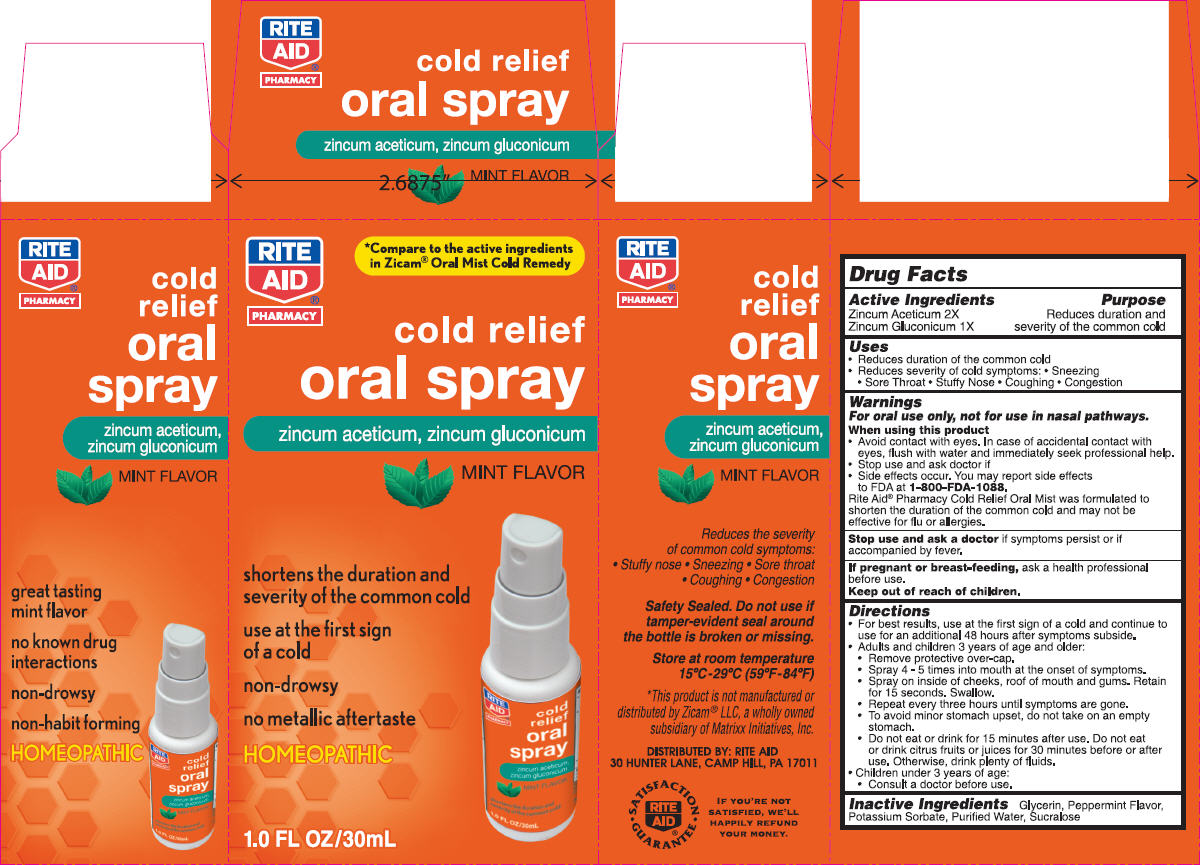
Label: NBE- zinc gluconate and zinc acetate spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 64762-871-30 - Packager: Dynamic Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 31, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For oral use only, not for use in nasal pathways.
When using this product
- Avoid contact with eyes. In case of accidental contact with eyes, flush with water and immediately seek professional help.
- Stop use and ask doctor if
- Side effects occur. You may report side effects to FDA at 1-800-FDA-1088.
Rite Aid® Pharmacy Cold Relief Oral Mist was formulated to shorten the duration of the common cold and may not be effective for flu or allergies.
-
Directions
- For best results, use at the first sign of a cold and continue to use for an additional 48 hours after symptoms subside.
- Adults and children 3 years of age and older:
- Remove protective over-cap.
- Spray 4 - 5 times into mouth at the onset of symptoms.
- Spray on inside of cheeks, roof of mouth and gums. Retain for 15 seconds. Swallow.
- Repeat every three hours until symptoms are gone.
- To avoid minor stomach upset, do not take on an empty stomach.
- Do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- Children under 3 years of age:
- Consult a doctor before use.
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
NBE
zinc gluconate and zinc acetate spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64762-871 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC - UNII:J41CSQ7QDS) ZINC GLUCONATE 12.26 mg in 1 mL ZINC ACETATE (UNII: FM5526K07A) (ZINC - UNII:J41CSQ7QDS) ZINC ACETATE 0.46 mg in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Potassium Sorbate (UNII: 1VPU26JZZ4) Water (UNII: 059QF0KO0R) Sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64762-871-30 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 09/30/2010 Labeler - Dynamic Pharmaceuticals Inc. (617660712) Establishment Name Address ID/FEI Business Operations Dynamic Pharmaceuticals Inc. 617660712 MANUFACTURE