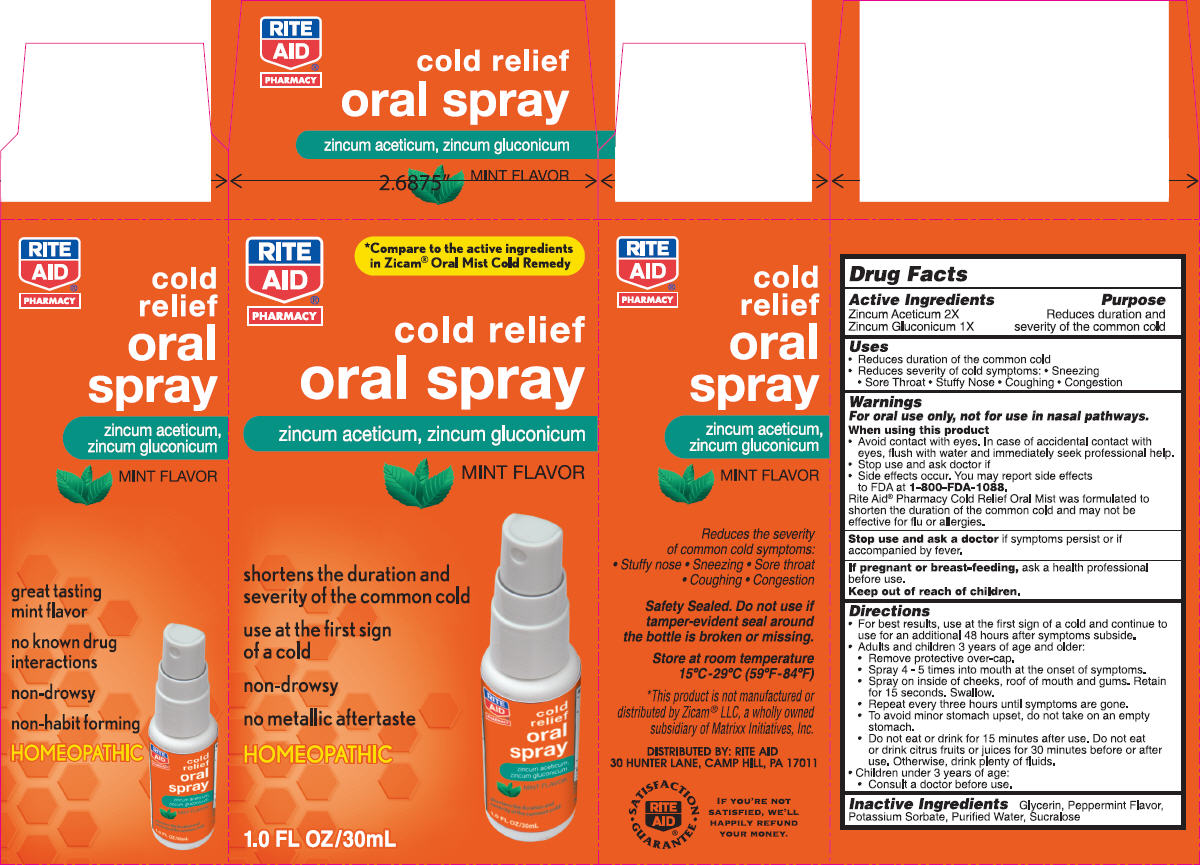
Uses
- Reduces duration of the common cold
- Reduces severity of cold symptoms:
- Sneezing
- Sore Throat
- Stuffy Nose
- Coughing
- Congestion
Warnings
For oral use only, not for use in nasal pathways.
When using this product
- Avoid contact with eyes. In case of accidental contact with eyes, flush with water and immediately seek professional help.
- Stop use and ask doctor if
- Side effects occur. You may report side effects to FDA at 1-800-FDA-1088.
Rite Aid® Pharmacy Cold Relief Oral Mist was formulated to shorten the duration of the common cold and may not be effective for flu or allergies.
Directions
- For best results, use at the first sign of a cold and continue to use for an additional 48 hours after symptoms subside.
- Adults and children 3 years of age and older:
- Remove protective over-cap.
- Spray 4 - 5 times into mouth at the onset of symptoms.
- Spray on inside of cheeks, roof of mouth and gums. Retain for 15 seconds. Swallow.
- Repeat every three hours until symptoms are gone.
- To avoid minor stomach upset, do not take on an empty stomach.
- Do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- Children under 3 years of age:
- Consult a doctor before use.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
RITE
AID®
PHARMACY
*Compare to the active ingredients
in Zicam® Oral Mist Cold Remedy
cold relief
oral spray
zincum aceticum, zincum gluconicum
MINT FLAVOR
shortens the duration and
severity of the common cold
use at the first sign
of a cold
non-drowsy
no metallic aftertaste
HOMEOPATHIC
1.0 FL OZ/30mL