Label: INDIGO CARMINE injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 50090-4520-0 - Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 0517-0375
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each mL contains: Indigotindisulfonate Sodium 8 mg, Water for Injection q.s. pH adjusted, when necessary, with Citric Acid and/or Sodium Citrate. Sterile, nonpyrogenic.
Sufficient Indigo Carmine is contained in each 5 mL ampule to permit accurate withdrawal and administration of the full dose. It gives a deep blue solution when dissolved in water.
The structural formula is:
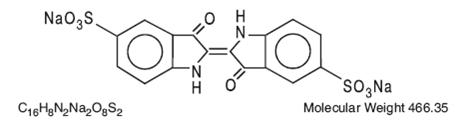
-
CLINICAL PHARMACOLOGY
Indigo Carmine is excreted largely by the kidneys, retaining its blue color during passage through the body.
Elimination of the dye begins soon after injection, appearing in the urine within 10 minutes in average cases. The biological half-life is 4 to 5 minutes following intravenous injection. Larger quantities are necessary when intramuscular injection is employed. Appearance time and elimination are delayed following intramuscular injection.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Pregnancy
Animal Reproduction studies have not been conducted with indigotindisulfonate sodium injection. It is also not known whether indigotindisulfonate sodium injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indigotindisulfonate sodium injection should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indigo Carmine is administered to a nursing woman.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Indigo Carmine solution is injected either by the intravenous or intramuscular route, and its appearance at the ureteral orifices is watched with the cystoscope in place. The intravenous method is preferred because a 5 mL injection is sufficient. A lesser dosage in infants, children and underweight patients will prevent skin coloration.
Since precipitation of indigotindisulfonate sodium may occur, Indigo Carmine Solution must not be diluted prior to injection or injected with infusion assemblies which were used with other solutions.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
NOTE: Since Indigo Carmine is a dark blue solution, visual inspection for particulate matter prior to use may not be possible. To ensure that the withdrawn solution contains no particulates, 5 micron filter straws/filter needles must be used when withdrawing contents of ampules1. The 5 micron nylon mesh filter is suitable for withdrawing the drug product, Indigo Carmine.
1 ASHP Guidelines on Compounding Sterile Preparations
PROTECT FROM LIGHT. Indigo Carmine should be stored in the dark, away from direct light, preferably in the original package.
Store at 20º to 25º C (68º to 77º F); excursions permitted to 15º to 30º C (59º to 86º F) (See USP Controlled Room Temperature).
- HOW SUPPLIED
- Indigo Carmine
-
INGREDIENTS AND APPEARANCE
INDIGO CARMINE
indigo carmine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50090-4520(NDC:0517-0375) Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDIGOTINDISULFONATE SODIUM (UNII: D3741U8K7L) (INDIGOTINDISULFONIC ACID - UNII:X7OI7JF73P) INDIGOTINDISULFONATE SODIUM 8 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50090-4520-0 5 mL in 1 AMPULE; Type 0: Not a Combination Product 09/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 09/30/1990 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-4520) , REPACK(50090-4520)


