Label: ARNICARE- arnica montana cream
- NDC Code(s): 0220-9021-60, 0220-9021-63, 0220-9021-68, 0220-9021-82
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- DO NOT USE
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
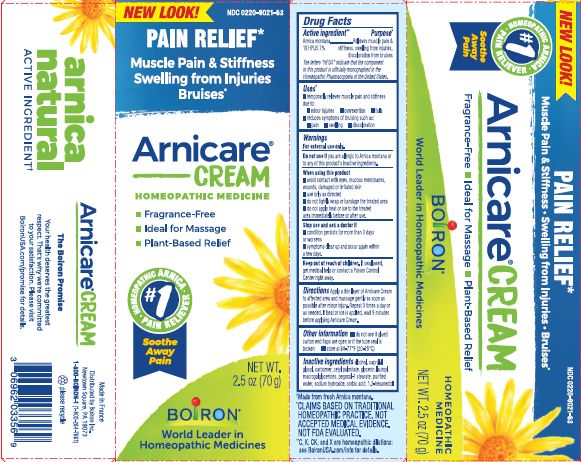
Arnicare Cream
Alcohol, Caprylyl glycol, carbomer, cetyl palmitate, EDTA disodium, glycerin, lauroyl macrogolglycerides, pegoxol-7 stearate, purified water, sodium hydroxide, sorbic acid, 1,2-hexanediol
Arnicare Cream (without disodium EDTA)
Alcohol, Caprylyl glycol, carbomer, cetyl palmitate, EDTA disodium, glycerin, lauroyl macrogolglycerides, pegoxol-7 stearate, purified water, sodium hydroxide, sorbic acid, 1,2-hexanediol
- HOW SUPPLIED
- PURPOSE
- STORAGE AND HANDLING
- QUESTIONS
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- WHEN USING
- DRUG INTERACTIONS
-
WARNINGS
For external use only
Avoid contact with eyes, mucous membranes, damaged skin or wounds.Do not use if you are allergic to Arnica montana or any of the this product's inactive ingredients.
When using this product, use only as directed: do not tightly wrap or bandage, do not apply heat or ice to the treated area immediately before or after use.
If swallowed, get medical help or contact a Poison Control Center right away.
-
SPL UNCLASSIFIED SECTION
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
ɨ Homeopathic mother tincture made from Arnica montana fresh whole plant.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICARE
arnica montana creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER 934 (UNII: Z135WT9208) CETYL PALMITATE (UNII: 5ZA2S6B08X) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBIC ACID (UNII: X045WJ989B) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) GLYCERIN (UNII: PDC6A3C0OX) PEGOXOL 7 STEARATE (UNII: 3EW5AXE5X5) ALCOHOL (UNII: 3K9958V90M) HYDROGENATED PALM/PALM KERNEL OIL PEG-6 ESTERS (UNII: 8EPU9MJ01K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9021-60 40 g in 1 TUBE; Type 0: Not a Combination Product 03/01/2007 2 NDC:0220-9021-63 70 g in 1 TUBE; Type 0: Not a Combination Product 03/01/2007 3 NDC:0220-9021-82 1 in 1 PACKAGE 06/01/2018 3 120 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:0220-9021-68 1 in 1 BOX 02/29/2016 4 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/01/2007 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9021)