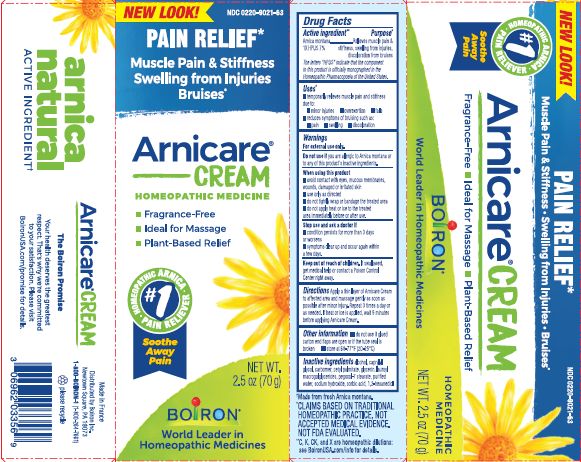
Temporarily relieves muscle pain and stiffness due to minor injuries, overexertion and falls. Reduces pain, swelling and discoloration from bruises.
Apply a thin layer of Arnicare Cream to affected area and massage after minor injury. Repeat 3 times a day or as needed.
Arnicare Cream
Alcohol, Caprylyl glycol, carbomer, cetyl palmitate, EDTA disodium, glycerin, lauroyl macrogolglycerides, pegoxol-7 stearate, purified water, sodium hydroxide, sorbic acid, 1,2-hexanediol
Arnicare Cream (without disodium EDTA)
Alcohol, Caprylyl glycol, carbomer, cetyl palmitate, EDTA disodium, glycerin, lauroyl macrogolglycerides, pegoxol-7 stearate, purified water, sodium hydroxide, sorbic acid, 1,2-hexanediol
Arnica Montana 1X ... Trauma, muscle pain and stiffness, swelling from injuries, discoloration from bruising.
Questions, Comments?
www.arnicare.com
www.boironusa.com
info@boironusa.com
1-800-BOIRON-1 (1-800-264-7661)
Distributed by Boiron Inc.
Newtown Square, PA 19073-3267
For external use only
Avoid contact with eyes, mucous membranes, damaged skin or wounds.
Do not use if you are allergic to Arnica montana or any of the this product's inactive ingredients.
When using this product, use only as directed: do not tightly wrap or bandage, do not apply heat or ice to the treated area immediately before or after use.
If swallowed, get medical help or contact a Poison Control Center right away.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
ɨ Homeopathic mother tincture made from Arnica montana fresh whole plant.