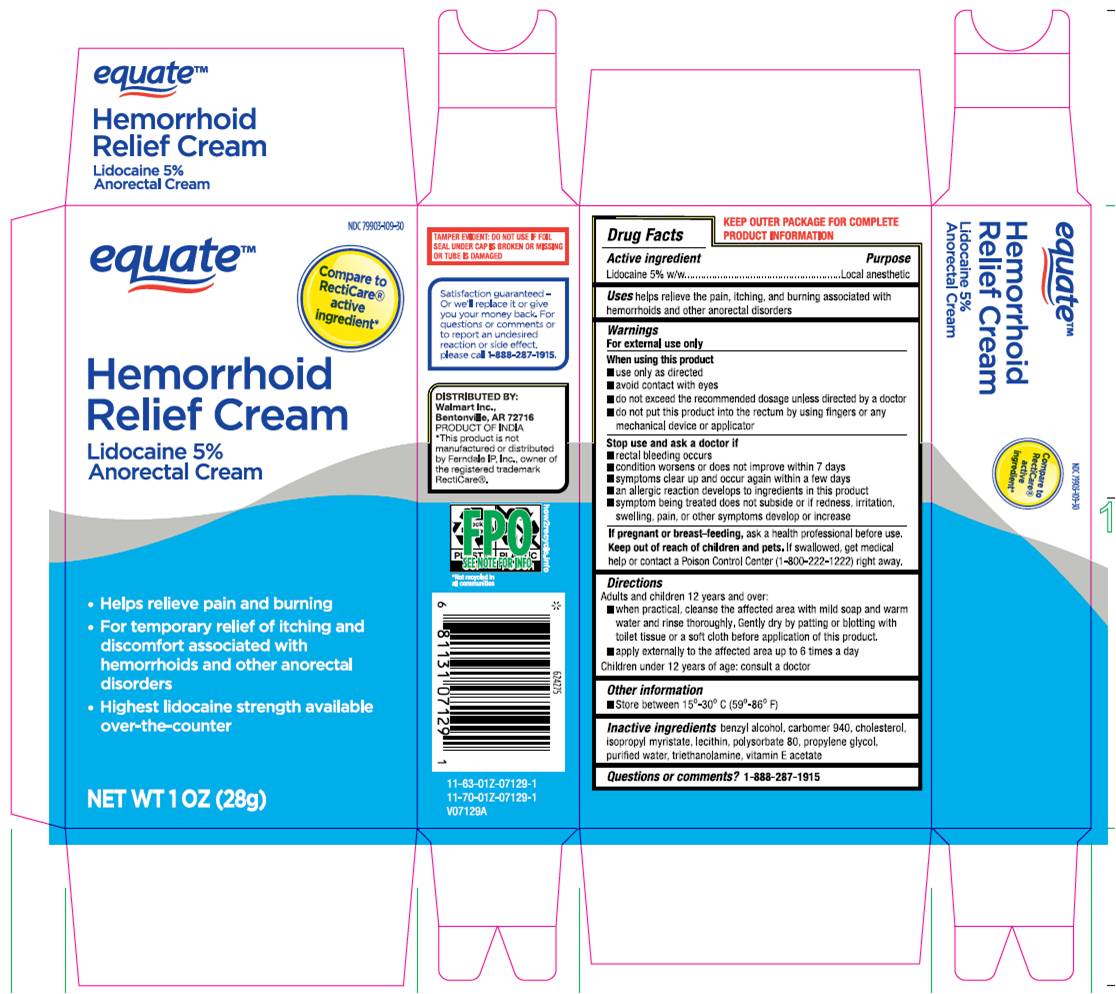
Label: HEMORRHOID RELIEF CREAM- lidocaine 5% anorectal cream
- NDC Code(s): 73492-712-28
- Packager: ALAINA HEALTHCARE PRIVATE LIMITED
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 11, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed.
- Avoid contact with eyes.
- do not exceed recommended dosage unless directed by a doctor.
- do not out this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if - rectal bleeding occurs.
- condition worsens or does not improve within 7 days.
- symptoms clear up and occur again within a few days.
- an allergic reaction develops to ingredients in this product.
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase.
If pregnant or breast-feeding,ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adult and children 12 years and over:
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly, gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- apply externally to the affected area up to 6 times a day children under 12 years of age: consult a doctor.
- Other Information.
- Inactive Ingredient
- Questions or Comments? 1-888-287-1915
-
SPL UNCLASSIFIED SECTION
Manufactured By:
Alaina Healthcare Private Limited
Khasra No. 127-133, Baddi-Barotiwala Road,
Baddi, HIMACHAL PRADESH 173205, India (IND).Distributed By:
Walmart Inc., Bentonville, AR 72716. PRODUCT OF INDIA.*This product is not manufactured or distributed by Ferndale IP, Inc., Owner of the registered trademark RectiCare ®.
- Package Label.Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HEMORRHOID RELIEF CREAM
lidocaine 5% anorectal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73492-712 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER 940 (UNII: 4Q93RCW27E) CHOLESTEROL (UNII: 97C5T2UQ7J) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73492-712-28 1 in 1 CARTON 01/28/2022 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 01/28/2022 Labeler - ALAINA HEALTHCARE PRIVATE LIMITED (858720927) Establishment Name Address ID/FEI Business Operations ALAINA HEALTHCARE PRIVATE LIMITED 858720927 manufacture(73492-712)