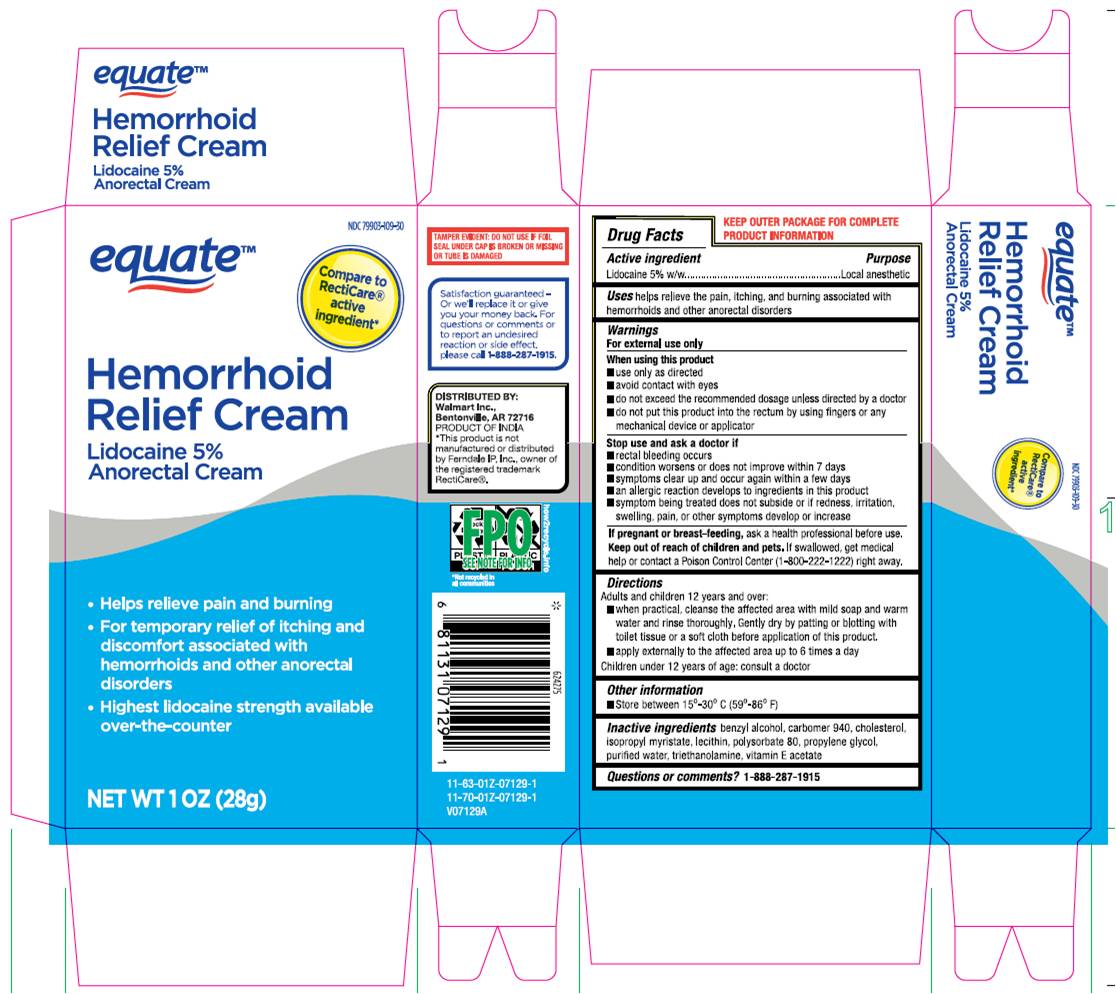
Uses
helps relieve the pain, itching, and burning, associated with hemorrhoids and anorectal disorders.
Warnings
For external use only
When using this product
- use only as directed.
- Avoid contact with eyes.
- do not exceed recommended dosage unless directed by a doctor.
- do not out this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if - rectal bleeding occurs.
- condition worsens or does not improve within 7 days.
- symptoms clear up and occur again within a few days.
- an allergic reaction develops to ingredients in this product.
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase.
If pregnant or breast-feeding,ask a health professional before use.
keep out of reach of children and pets.If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Adult and children 12 years and over:
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly, gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- apply externally to the affected area up to 6 times a day children under 12 years of age: consult a doctor.
Inactive Ingredient
Benzyl Alcohol, Carbomer 940, Cholesterol, Isopropyl Myristate, Lecithin (Soya), Polysorbate 80, Propylene Glycol, Purified Water, Triethanolamine, Vitamin-E Acetate.
Manufactured By:
Alaina Healthcare Private Limited
Khasra No. 127-133, Baddi-Barotiwala Road,
Baddi, HIMACHAL PRADESH 173205, India (IND).
Distributed By:
Walmart Inc., Bentonville, AR 72716. PRODUCT OF INDIA.
*This product is not manufactured or distributed by Ferndale IP, Inc., Owner of the registered trademark RectiCare ®.