Label: ALKA-SELTZER PLUS MAXIMUM STRENGTH SEVERE SINUS, ALLERGY AND COUGH- acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, phenylephrine hydrochloride capsule, liquid filled
- NDC Code(s): 0280-1620-20
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
-
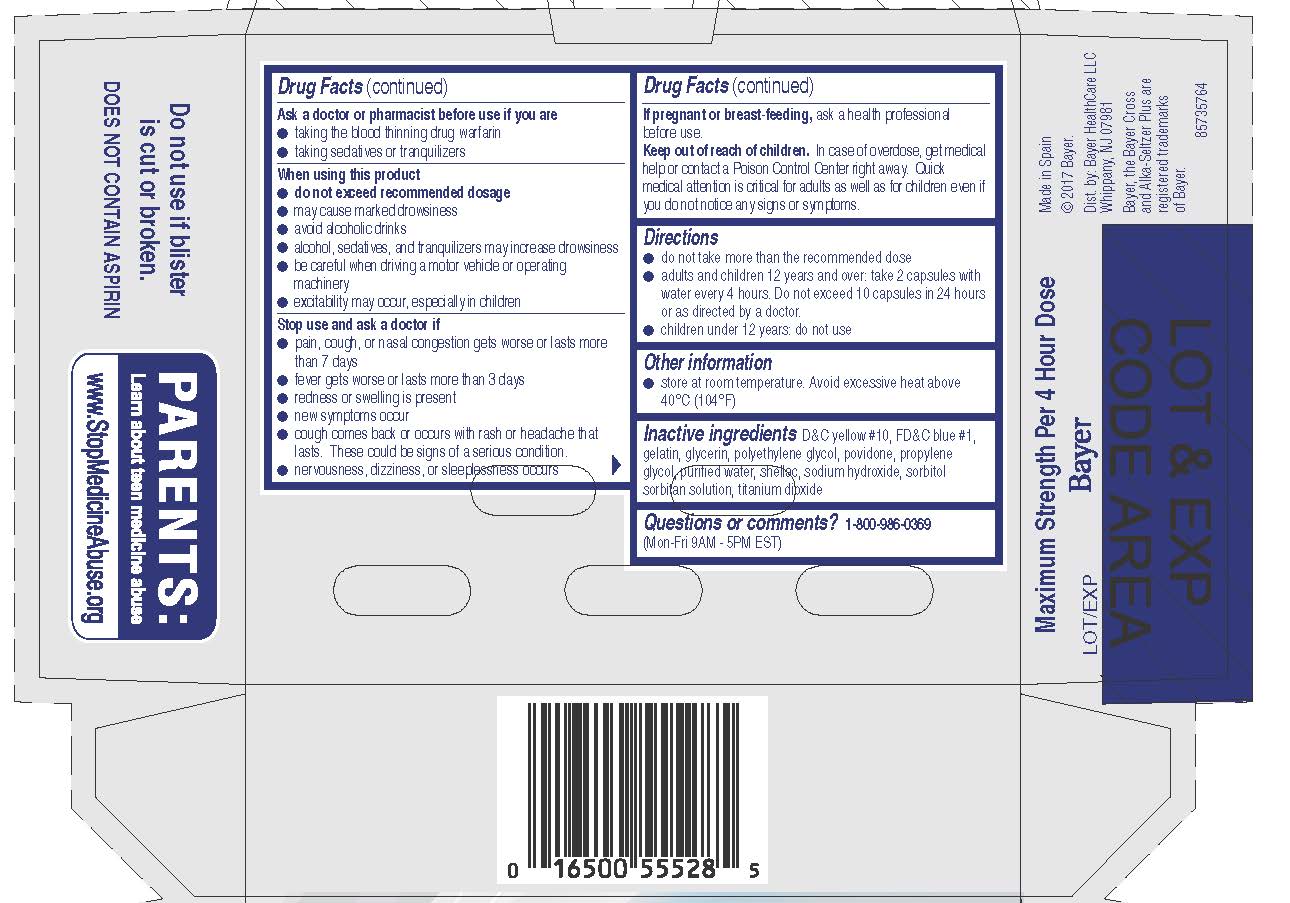
Uses
Uses
· temporarily relieves these symptoms due to hay fever or other upper
respiratory allergies:
· runny nose · sneezing
· itching of the nose or throat · itchy, watery eyes
· temporarily relieves these symptoms due to a cold:
· nasal congestion · sinus congestion and pressure
· headache · minor aches and pains · cough
· temporarily reduces fever
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Do not use to sedate children.
-
DO NOT USE
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
-
ASK DOCTOR
Ask a doctor before use if you have
● liver disease ● heart disease ● high blood pressure
● thyroid disease ● diabetes ● glaucoma
● cough with excessive phlegm (mucus)
● a breathing problem such as emphysema or chronic bronchitis
● difficulty in urination due to enlargement of the prostate gland
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
· pain, cough, or nasal congestion gets worse or lasts more than
7 days
· fever gets worse or lasts more than 3 days
· redness or swelling is present
· new symptoms occur
· cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
· nervousness, dizziness, or sleeplessness occurs
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALKA-SELTZER PLUS MAXIMUM STRENGTH SEVERE SINUS, ALLERGY AND COUGH
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, phenylephrine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-1620 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITOL (UNII: 506T60A25R) SORBITAN (UNII: 6O92ICV9RU) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color green Score no score Shape OVAL Size 20mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-1620-20 2 in 1 CARTON 06/26/2017 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/26/2017 Labeler - Bayer HealthCare LLC. (112117283)