Label: NINJACOF-D- chlophedianol hydrochloride, pyrilamine maleate, pseudoephedrine hydrochloride liquid
- NDC Code(s): 23359-034-04, 23359-034-16
- Packager: Centurion Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses:
-
WARNINGS
Do not exceed recommended dosage
Do not use this product if
- you are now taking prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
-
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- a cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- Ask doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If Pregnant or breast feeding
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- Questions? Comments?
-
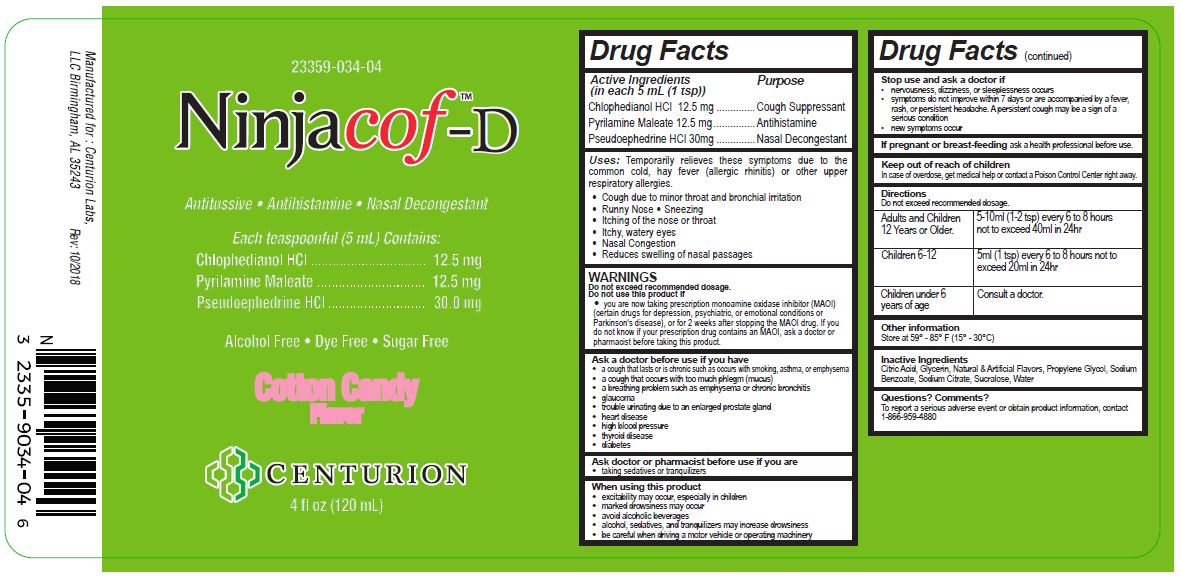
PRINCIPAL DISPLAY PANEL

23359-034-16
Ninjacof ™-D
Antitussive, Antihistamine, Nasal Decongestant
Each teaspoonful (5mL) Contains:
Chlophedianol HCl........................................12.5 mg
Pyrilamine Maleate.......................................12.5 mg
Pseudoephedrine HCl....................................30.0 mg
Alchohol Free, Dye Free, Sugar Free
Cotton Candy Flavor
Centurion
16 fl oz (473 mL)
Manufactured for: Centurion Labs, LLC Birmingham, AL 35243
Rev 07/2017
-
PRINCIPAL DISPLAY PANEL
23359-034-04
Ninjacof™-D
Antitussive, Antihistamine, Nasal Decongestant
Each Teaspoonful (5 mL) Contains:
Chlohedianol HCl......................................12.5 mg
Pyrilamine Maleate...................................12.5 mg
Pseudoephedrine HCl................................30.0 mg
Alcohol Free, Dye Free, Sugar Free
Cotton Candy Flavor
Centurion
4 fl oz (120 mL)
Manufactured for: Centurion Labs, LLC Birmingham, AL 35243
Rev. 10/2018
-
INGREDIENTS AND APPEARANCE
NINJACOF-D
chlophedianol hydrochloride, pyrilamine maleate, pseudoephedrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23359-034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 12.5 mg in 5 mL PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 30 mg in 5 mL CHLOPHEDIANOL HYDROCHLORIDE (UNII: 69QQ58998Y) (CHLOPHEDIANOL - UNII:42C50P12AP) CHLOPHEDIANOL HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID ACETATE (UNII: DSO12WL7AU) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor COTTON CANDY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23359-034-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/16/2017 2 NDC:23359-034-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/16/2017 Labeler - Centurion Labs, LLC (016481957)