Drug Facts
Active Ingredients (in each 5 mL (1 tsp)) Purpose
Chlophedianol HCl 12.5 mg...........................Cough Suppressant
Pyrilamine Maleate 12.5 mg...........................Antihistamine
Pseudoephedrine HCl 30 mg...........................Nasal Decongestant
Uses:
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies.
- Cough due to minor throat and bronchial irritation
- Runny Nose
- Sneezing
- Itching of the nose or throat
- Itchy, watery eyes
- Nasal Congestion
- Reduces swelling of nasal passages
WARNINGS
Do not exceed recommended dosage
Do not use this product if
- you are now taking prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- a cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- heart disease
- high blood pressure
- thyroid disease
- diabetes
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occurs
- symptoms do not improve within 7 days or are accompanied by a fever,
- rash, or persistent headache. A persistent cough may be a sign of a serious condition
- new symptoms occur
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Do not exceed recommended dosage.
| Adults and Children
12 Years or Older. | 5-10ml (1-2 tsp) every 6 to 8 hours
not to exceed 40ml in 24hr |
| Children 6-12 | 5ml (1 tsp) every 6 to 8 hours not to
exceed 20ml in 24hr |
| Children under 6
years of age | Consult a doctor. |
Inactive Ingredients
Citric Acid, Glycerin, Natural & Artificial Flavors, Propylene Glycol, Sodium Benzoate, Sodium Citrate, Sucralose, Water
Questions? Comments?
To report a serious adverse event or obtain product information, contact 1-866-959-4880
PRINCIPAL DISPLAY PANEL

23359-034-16
Ninjacof ™-D
Antitussive, Antihistamine, Nasal Decongestant
Each teaspoonful (5mL) Contains:
Chlophedianol HCl........................................12.5 mg
Pyrilamine Maleate.......................................12.5 mg
Pseudoephedrine HCl....................................30.0 mg
Alchohol Free, Dye Free, Sugar Free
Cotton Candy Flavor
Centurion
16 fl oz (473 mL)
Manufactured for: Centurion Labs, LLC Birmingham, AL 35243
Rev 07/2017
PRINCIPAL DISPLAY PANEL
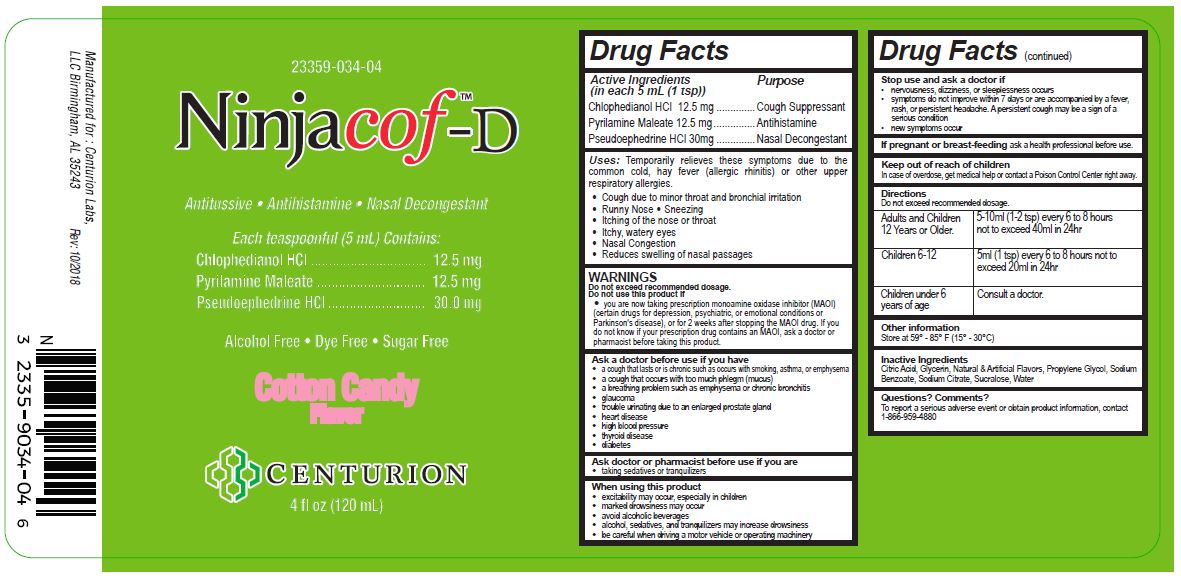
23359-034-04
Ninjacof™-D
Antitussive, Antihistamine, Nasal Decongestant
Each Teaspoonful (5 mL) Contains:
Chlohedianol HCl......................................12.5 mg
Pyrilamine Maleate...................................12.5 mg
Pseudoephedrine HCl................................30.0 mg
Alcohol Free, Dye Free, Sugar Free
Cotton Candy Flavor
Centurion
4 fl oz (120 mL)
Manufactured for: Centurion Labs, LLC Birmingham, AL 35243
Rev. 10/2018