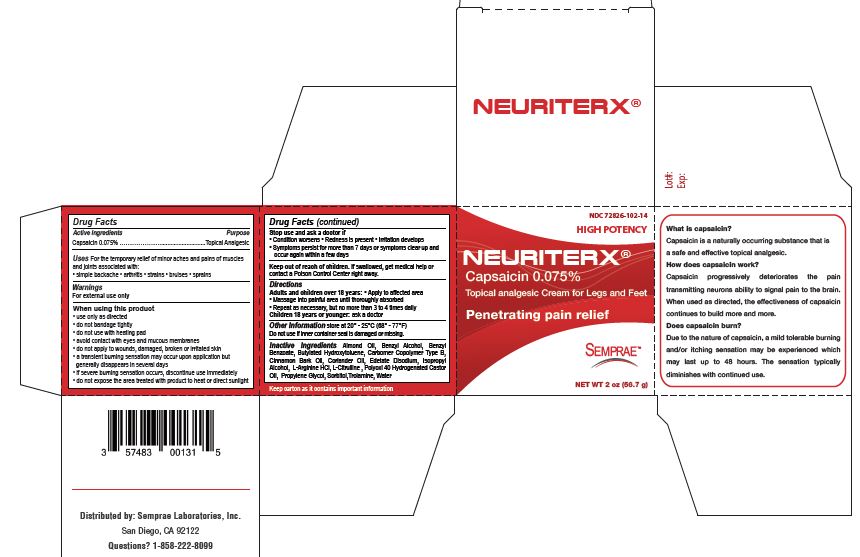
Label: NEURITRX- capsaicin 0.075% cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72826-102-14 - Packager: Semprae Laboratories Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 24, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
-
When using this product
• use only as directed
• do not bandage tightly
• do not use with heating pad
• avoid contact with eyes and mucous membranes
• do not apply to wounds, damaged, broken or irritated skin
• a transient burning sensation may occur upon application but generally disappears in several days
• if severe burning sensation occurs, discontinue use immediately
• do not expose the area treated with product to heat or direct sunlight - Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- Neuritrx carton
-
INGREDIENTS AND APPEARANCE
NEURITRX
capsaicin 0.075% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72826-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN .075 g in 100 g Inactive Ingredients Ingredient Name Strength ALMOND OIL (UNII: 66YXD4DKO9) ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZYL BENZOATE (UNII: N863NB338G) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) CITRULLINE (UNII: 29VT07BGDA) CINNAMON BARK OIL (UNII: XE54U569EC) CORIANDER OIL (UNII: 7626GC95E5) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72826-102-14 1 in 1 CARTON 11/29/2019 1 56.7 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/29/2019 Labeler - Semprae Laboratories Inc (093739668) Registrant - Semprae Laboratories Inc (093739668) Establishment Name Address ID/FEI Business Operations Topical Pharmaceuticals Inc. 831530683 manufacture(72826-102)