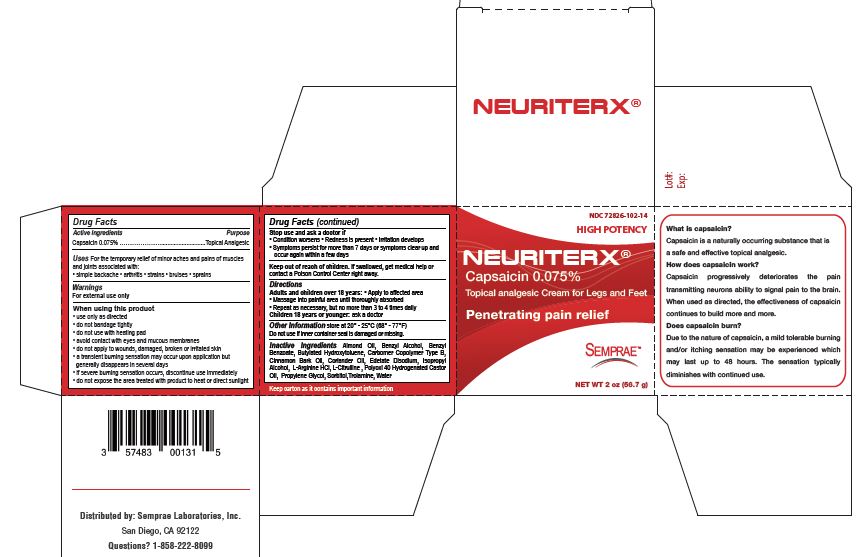
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with:
• simple backache
• arthritis
• strains
• bruises
• sprains
When using this product
• use only as directed
• do not bandage tightly
• do not use with heating pad
• avoid contact with eyes and mucous membranes
• do not apply to wounds, damaged, broken or irritated skin
• a transient burning sensation may occur upon application but generally disappears in several days
• if severe burning sensation occurs, discontinue use immediately
• do not expose the area treated with product to heat or direct sunlight
Stop use and ask a doctor if
• Condition worsens
• Redness is present
• Irritation develops
• Symptoms persist for more than 7 days or symptoms clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children over 18 years:
• Apply to affected area
• Massage into painful area until thoroughly absorbed
• Repeat as necessary, but no more than 3 to 4 times daily
Children 18 years or younger: ask a doctor
Other Information
Store at 20° - 25°C (68° - 77°F)
Do not use if inner container seal is damaged or missing.
Inactive Ingredients
Almond Oil, Benzyl Alcohol, Benzyl Benzoate, Butylated Hydroxytoluene, Carbomer Copolymer Type B, Cinnamon Bark Oil, Coriander Oil, Edetate Disodium, Isopropyl Alcohol, L-Arginine HCl, L-Citrulline , Polyoxl 40 Hydrogenated Castor Oil, Propylene Glycol, Sorbitol,Trolamine, Water