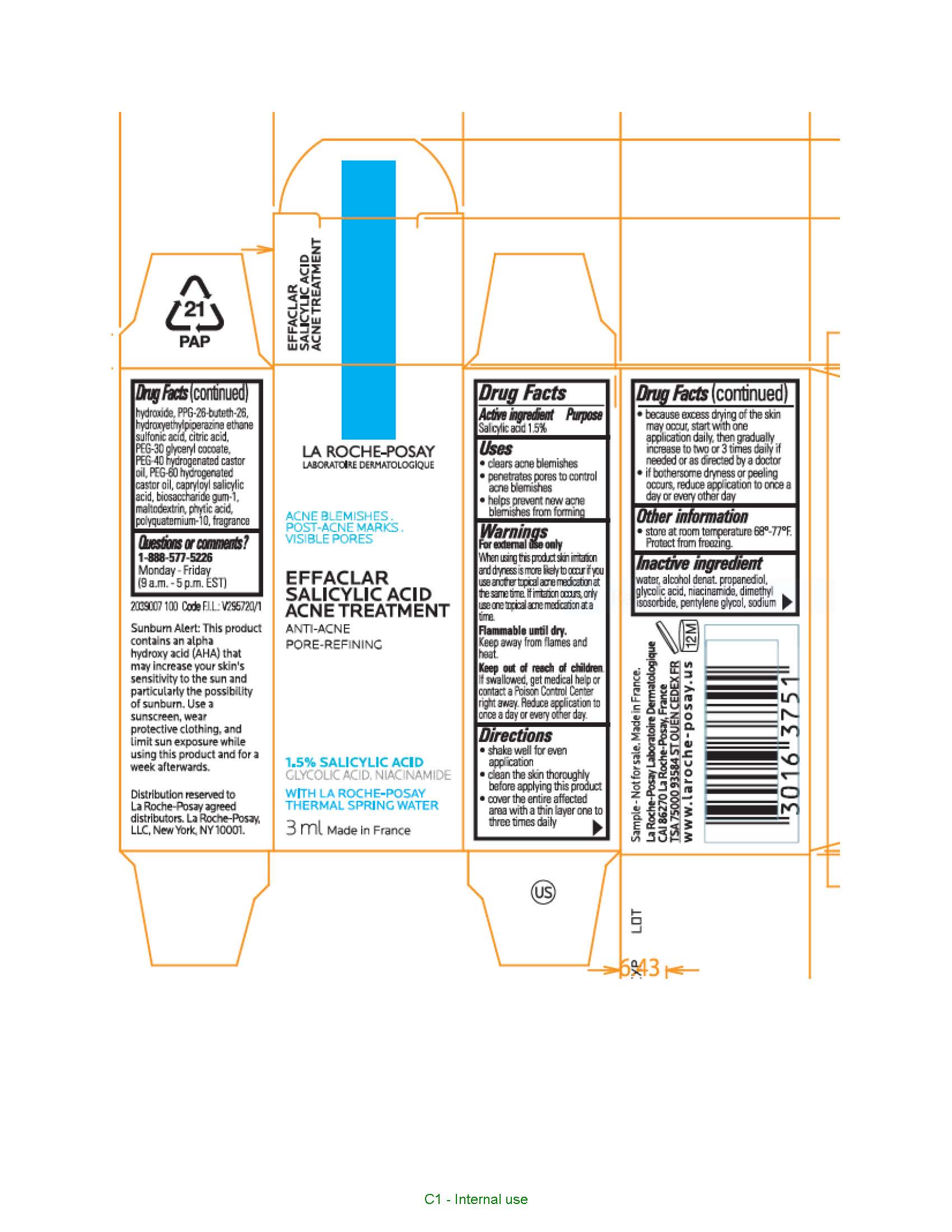
Label: LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE EFFACLAR PORE REFINING ACNE TREATMENT- salicylic acid liquid
- NDC Code(s): 49967-529-01, 49967-529-02
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- children under 12 years of age: ask a doctor
-
Inactive ingredients
water, alcohol denat., propanediol, glycolic acid, niacinamide, dimethyl isosorbide, pentylene glycol, sodium hydroxide, capitolize-26-buteth-26, PPG-26-buteth-26, hydroxyethylpiperazine ethane sulfonic acid, citric acid, PEG-30 glyceryl cocoate, PEG-40 hydrogenated castor oil, PEG-60 hydrogenated castor oil, capryloyl salicylic acid, biosaccharide gum-1, maltodextrin, phytic acid, polyquaternium-10, fragrance
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE EFFACLAR PORE REFINING ACNE TREATMENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-529 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) PROPANEDIOL (UNII: 5965N8W85T) GLYCOLIC ACID (UNII: 0WT12SX38S) NIACINAMIDE (UNII: 25X51I8RD4) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM HYDROXIDE (UNII: 55X04QC32I) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID (UNII: RWW266YE9I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PEG-30 GLYCERYL COCOATE (UNII: FX3EB7VC3L) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) CAPRYLOYL SALICYLIC ACID (UNII: 5F7PJF6AA4) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) MALTODEXTRIN (UNII: 7CVR7L4A2D) FYTIC ACID (UNII: 7IGF0S7R8I) POLYQUATERNIUM-10 (10000 MPA.S AT 2%) (UNII: PI1STR9QYH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-529-01 1 in 1 CARTON 05/01/2022 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:49967-529-02 1 in 1 CARTON 05/01/2022 2 3 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/01/2022 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations COSMETIQUE ACTIVE PRODUCTION 282658798 manufacture(49967-529) , pack(49967-529) Establishment Name Address ID/FEI Business Operations Interspray 364829903 pack(49967-529)