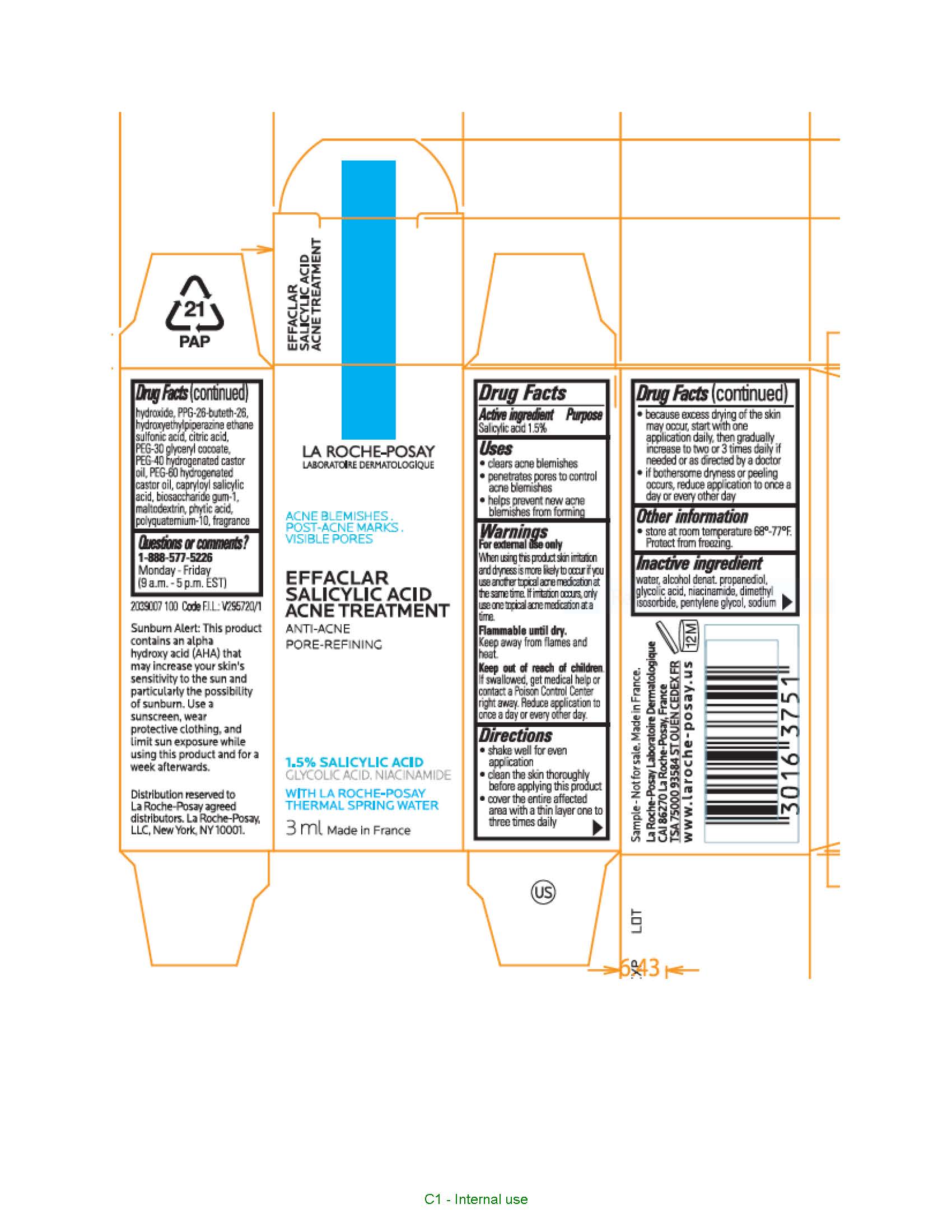
Uses
- clears acne blemishes
- penetrates pores to control acne blemishes
- helps prevent new acne blemishes from forming
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- children under 12 years of age: ask a doctor
Inactive ingredients
water, alcohol denat., propanediol, glycolic acid, niacinamide, dimethyl isosorbide, pentylene glycol, sodium hydroxide, capitolize-26-buteth-26, PPG-26-buteth-26, hydroxyethylpiperazine ethane sulfonic acid, citric acid, PEG-30 glyceryl cocoate, PEG-40 hydrogenated castor oil, PEG-60 hydrogenated castor oil, capryloyl salicylic acid, biosaccharide gum-1, maltodextrin, phytic acid, polyquaternium-10, fragrance