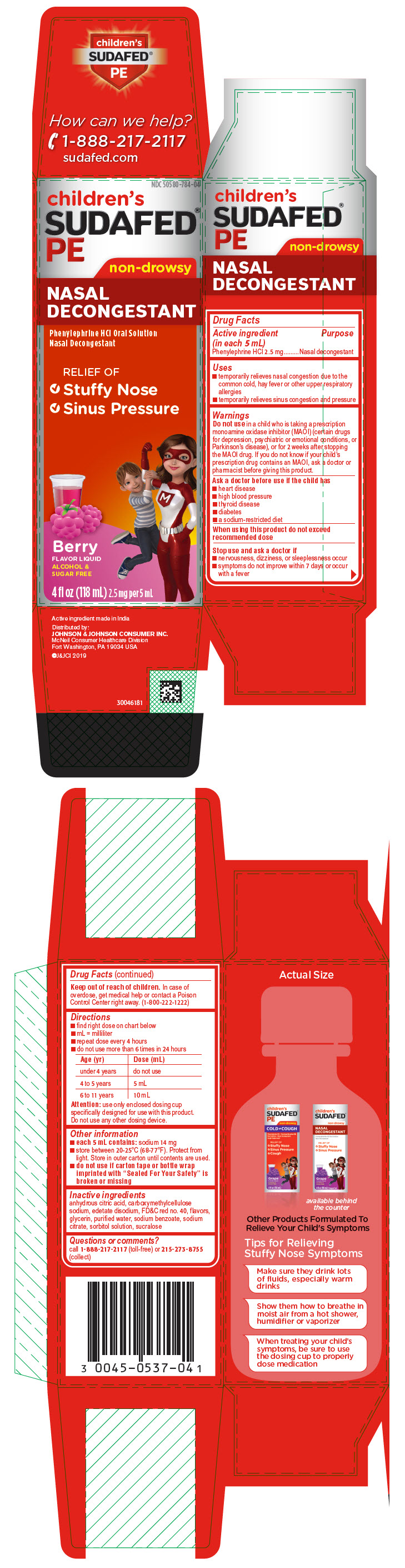
Label: CHILDRENS SUDAFED PE NASAL DECONGESTANT- phenylephrine hydrochloride solution
- NDC Code(s): 50580-784-04
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- a sodium-restricted diet
-
Directions
- find right dose on chart below
- mL = milliliter
- repeat dose every 4 hours
- do not use more than 6 times in 24 hours
Age (yr) Dose (mL) under 4 years do not use 4 to 5 years 5 mL 6 to 11 years 10 mL Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS SUDAFED PE NASAL DECONGESTANT
phenylephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-784 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL SOLUTION (UNII: 8KW3E207O2) SUCRALOSE (UNII: 96K6UQ3ZD4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color red Score Shape Size Flavor RASPBERRY (BERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-784-04 1 in 1 CARTON 10/01/2008 1 118 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/01/2008 Labeler - Johnson & Johnson Consumer Inc. (878046358)