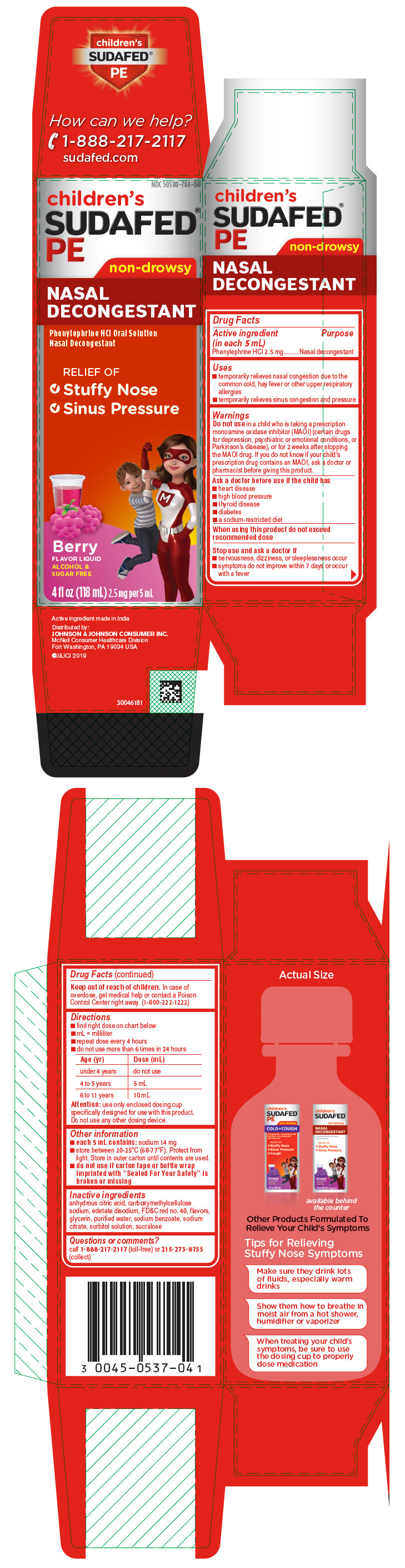
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- a sodium-restricted diet
Directions
- find right dose on chart below
- mL = milliliter
- repeat dose every 4 hours
- do not use more than 6 times in 24 hours
| Age (yr) | Dose (mL) |
|---|---|
| under 4 years | do not use |
| 4 to 5 years | 5 mL |
| 6 to 11 years | 10 mL |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
Other information
- each 5 mL contains: sodium 14 mg
- store between 20-25°C (68-77°F). Protect from light. Store in outer carton until contents are used.
- do not use if carton tape or bottle wrap imprinted with "Sealed For Your Safety" is broken or missing