Label: ECLIPSE SPF 50 ALL SHADES- titanium dioxide, zinc oxide lotion
-
NDC Code(s):
69219-102-01,
69219-102-02,
69219-102-03,
69219-102-11, view more69219-102-13, 69219-102-21, 69219-102-51, 69219-102-53
- Packager: SCIENCE OF SKINCARE LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- APPLY LIBERALLY 15 MINUTES BEFORE SUN EXPOSURE.
- REAPPLY:
- AFTER 40 MINUTES OF SWIMMING OR SWEATING
- IMMEDIATELY AFTER TOWEL DRYING
- AT LEAST EVERY 2 HOURS
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
o limit time in the sun, especially from 10 a.m.-2 p.m.
o wear long-sleeve shirts, pants, hats, and sunglasses
• Children under 6 months: Ask a doctor - Other Information
- Questions or Comments?
-
Inactive Ingredients
1,2-Hexanediol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Alumina, Butylene Glycol, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cetearyl Alcohol, Cetearyl Glucoside, Disodium EDTA, Dimethicone, Glyceryl Stearate, Methicone, Octyldodecyl Neopentanoate, PEG-100 Stearate, Polyhydroxystearic Acid, Sorbitan Laurate, Styrene/Acrylates Copolymer, Tocopherol, Tocopheryl Acetate, Triethoxycaprylylsilane, VP/Eicosene Copolymer, Water/Aqua/Eau, Xanthan Gum +/- Iron Oxides.
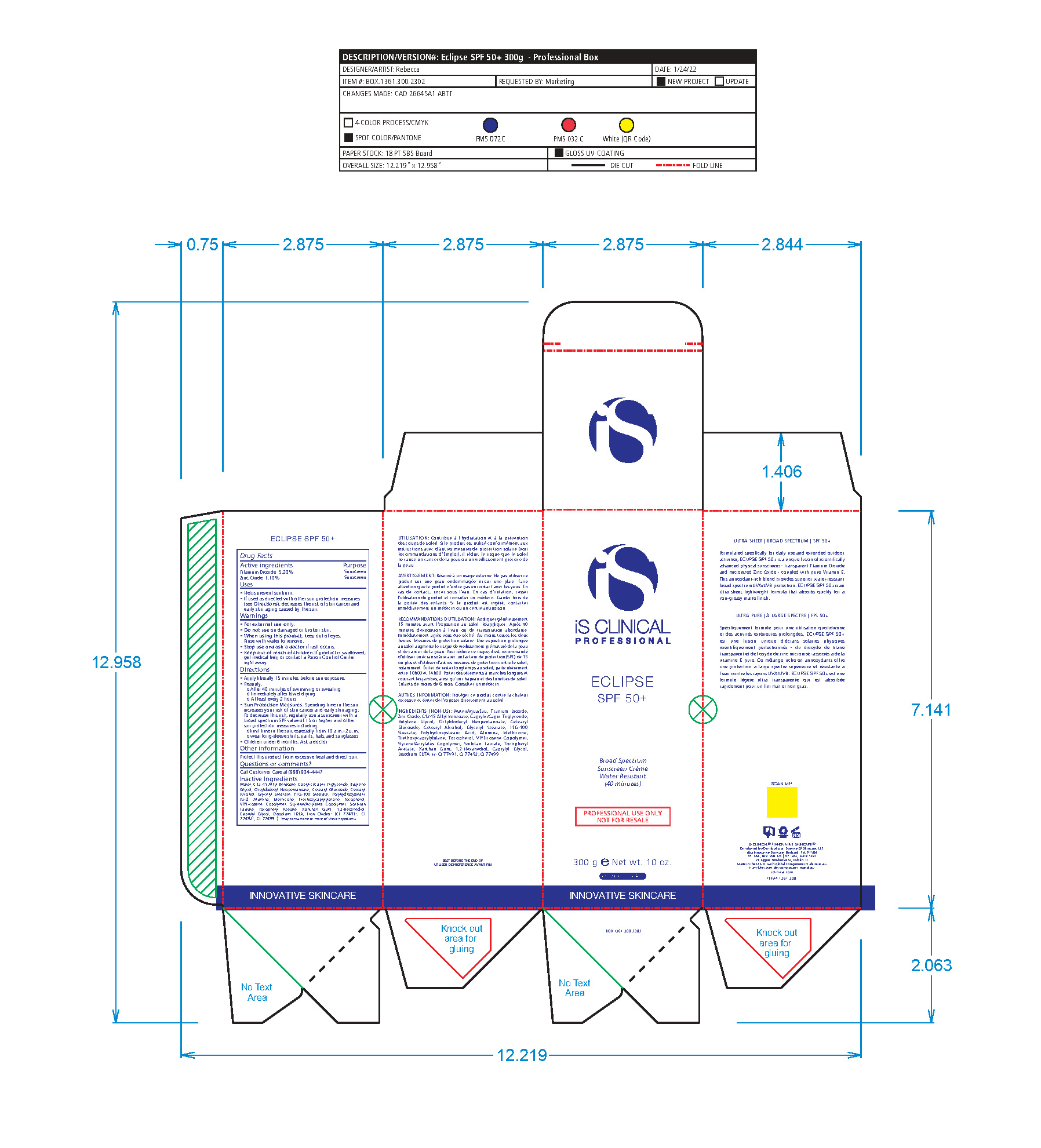
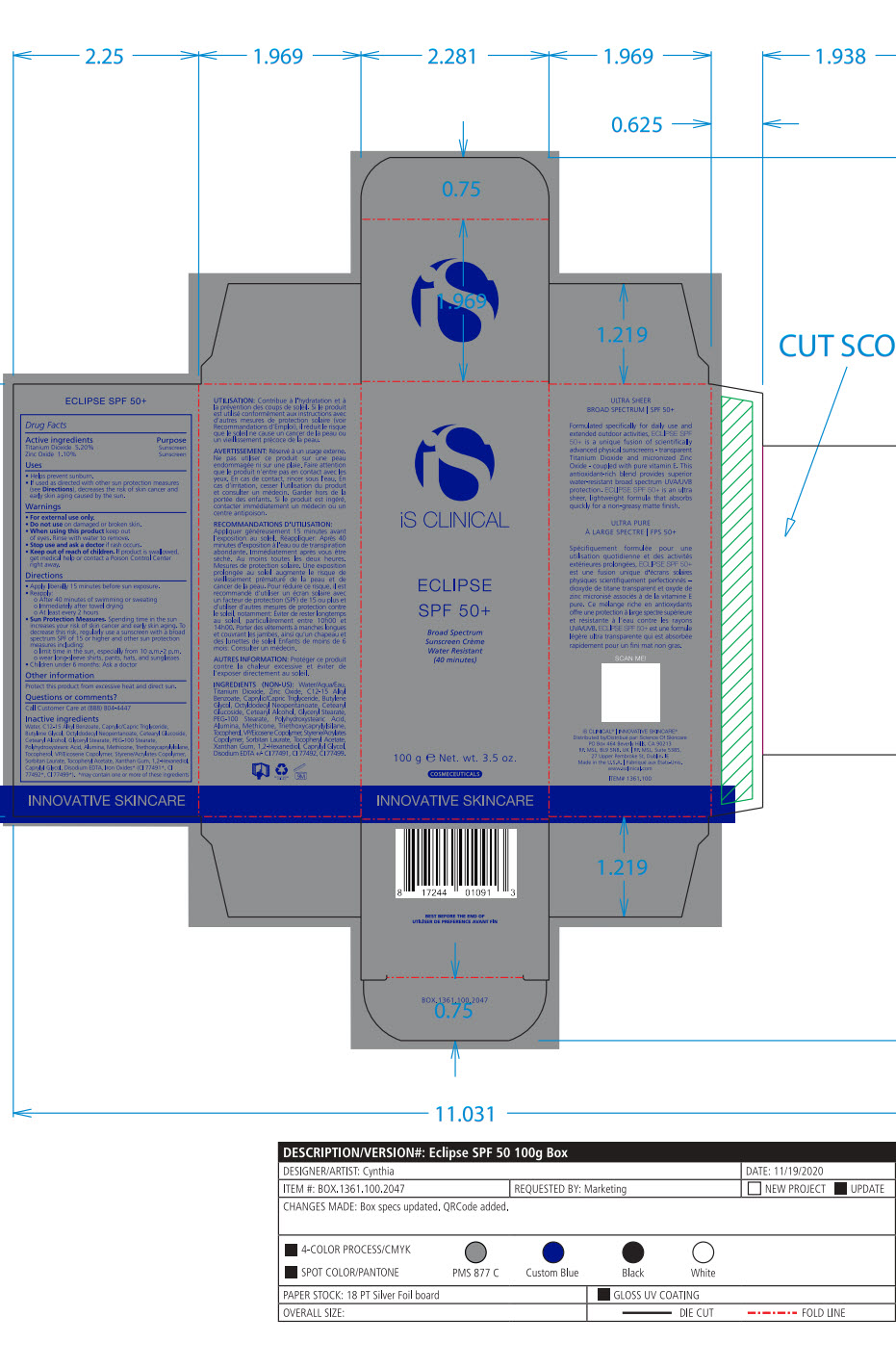
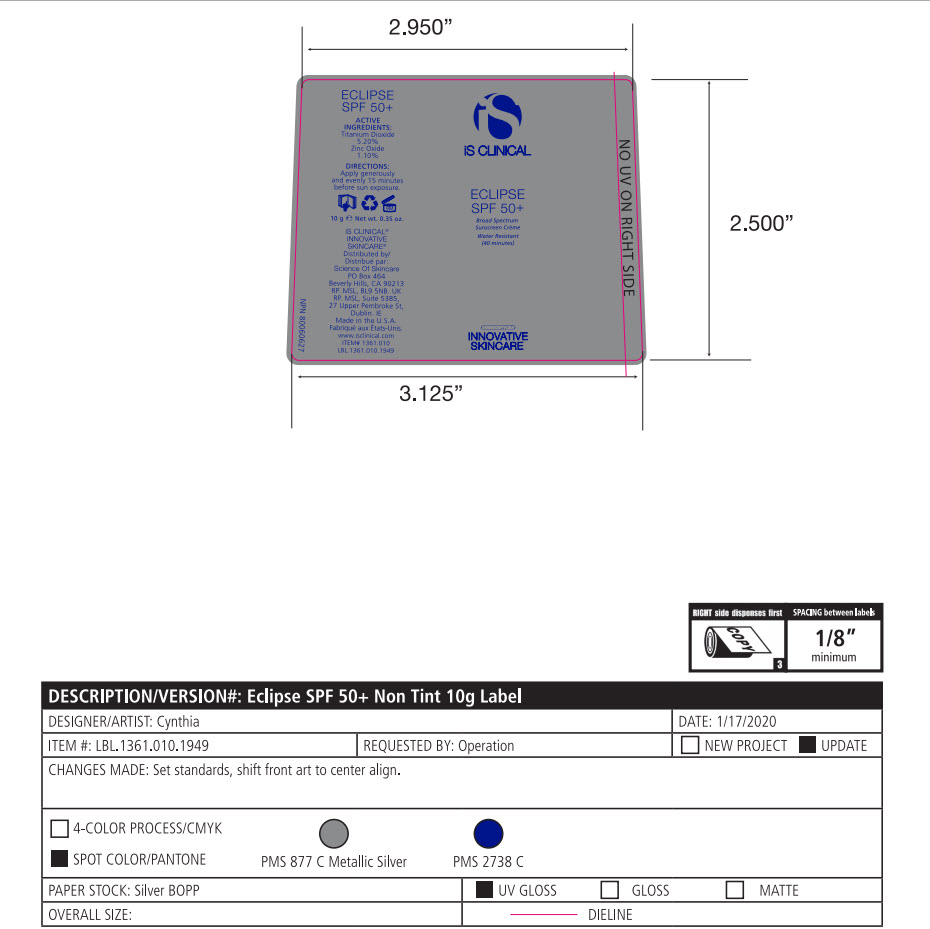
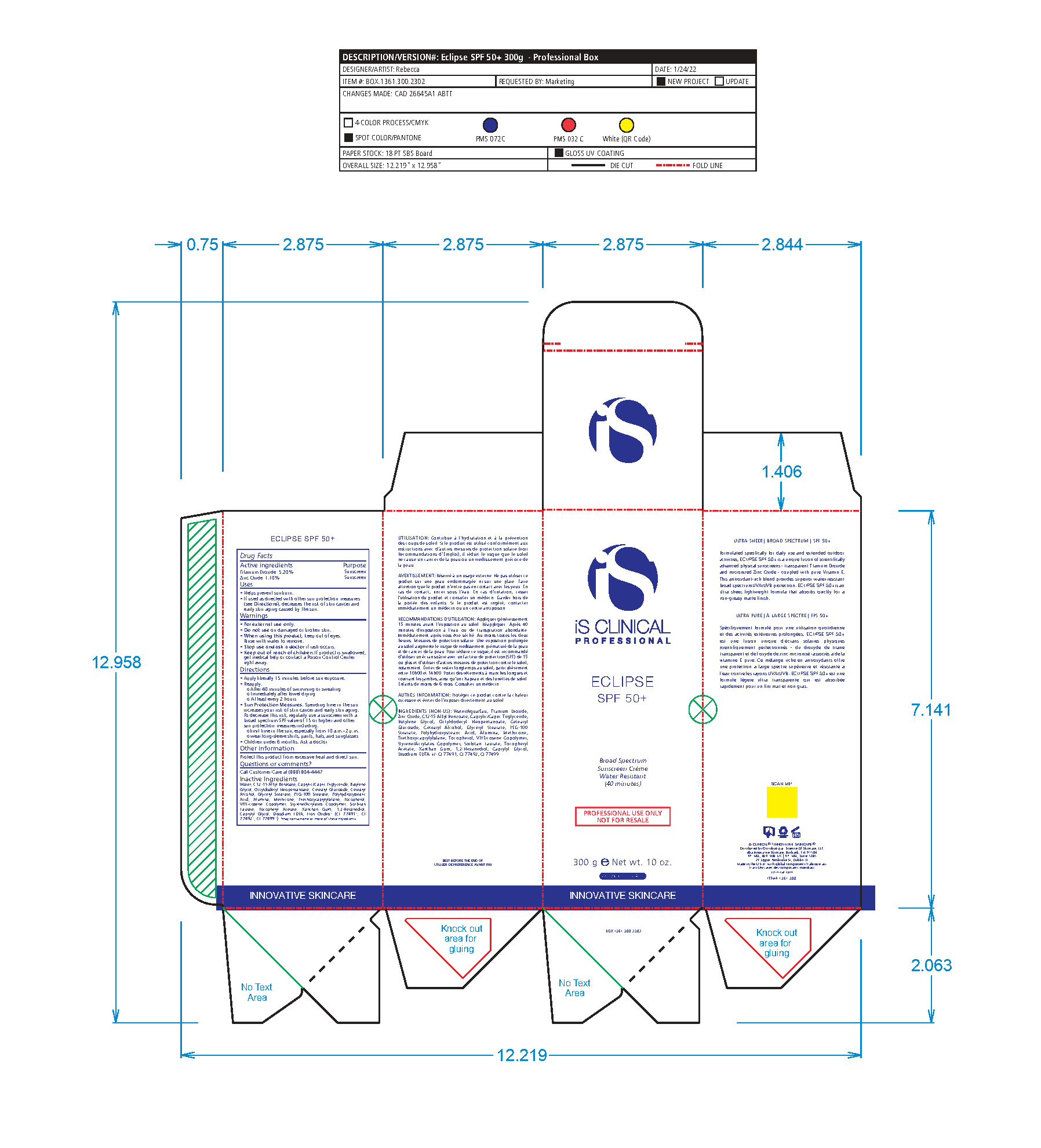
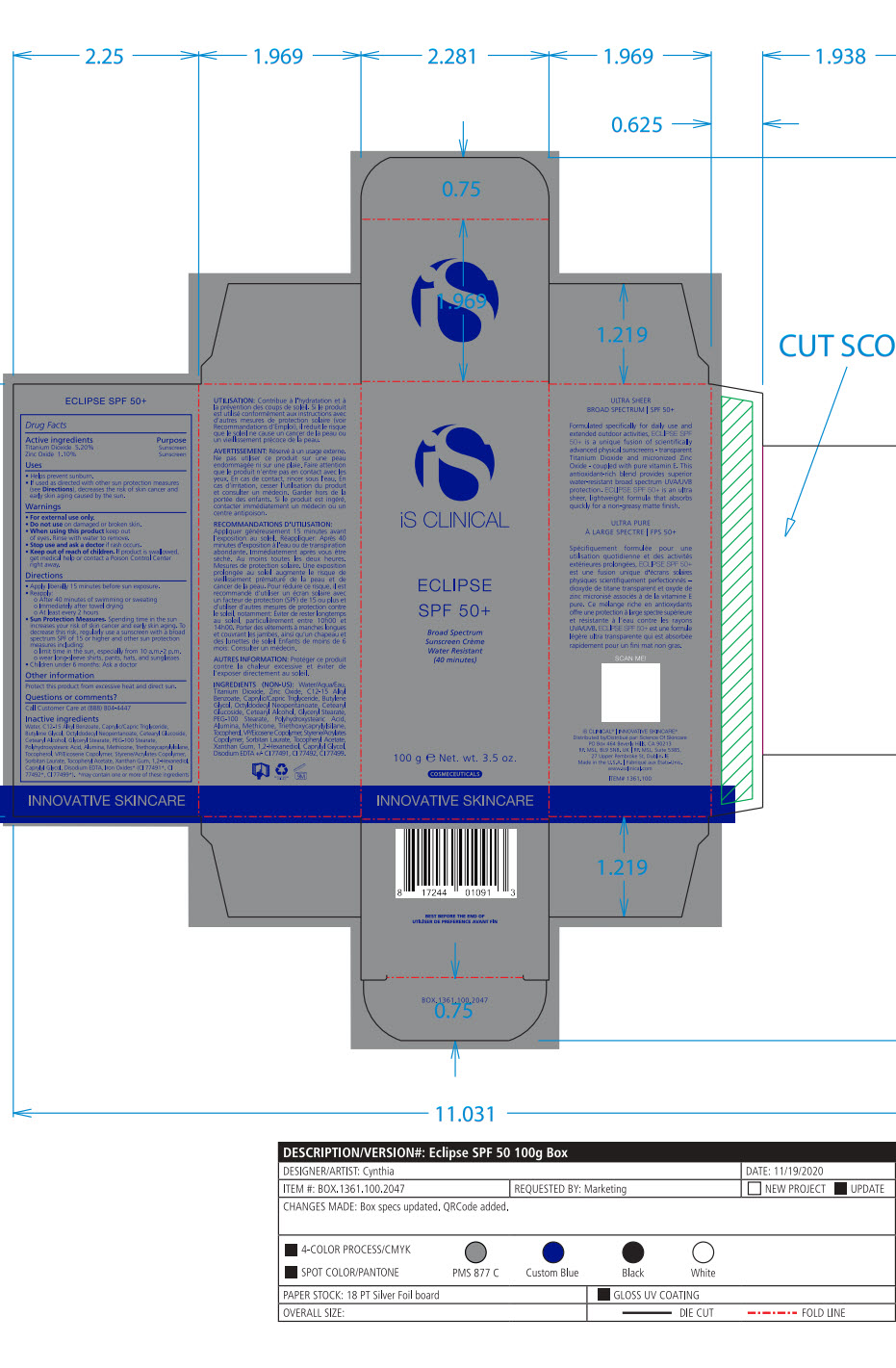
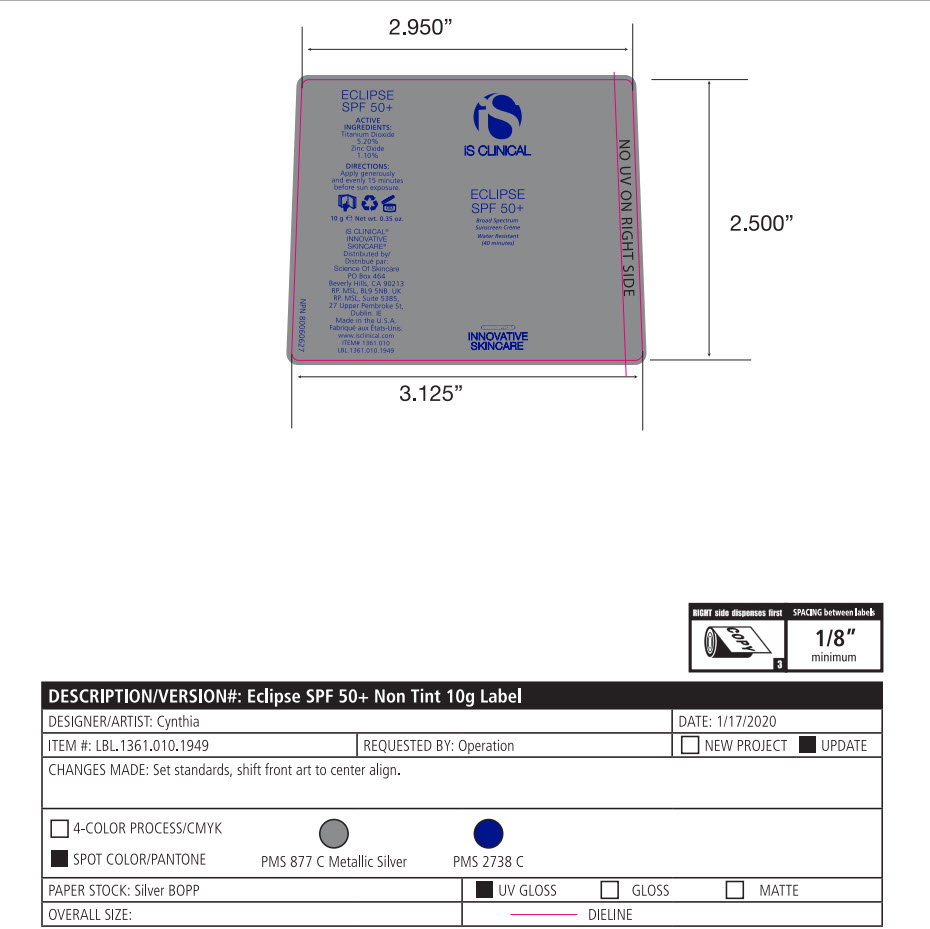
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ECLIPSE SPF 50 ALL SHADES
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69219-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.2 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 1.1 g in 100 g Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM (UNII: CPD4NFA903) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PEG-100 STEARATE (UNII: YD01N1999R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) STYRENE (UNII: 44LJ2U959V) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69219-102-51 1 in 1 BOX 10/01/2014 1 NDC:69219-102-13 90 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:69219-102-11 1 in 1 BOX 10/01/2014 2 NDC:69219-102-01 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC:69219-102-21 1 in 1 BAG 10/01/2014 3 NDC:69219-102-02 10 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:69219-102-03 300 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2014 5 NDC:69219-102-01 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2014 6 NDC:69219-102-02 10 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2014 7 NDC:69219-102-53 1 in 1 BOX 08/01/2023 7 300 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2014 Labeler - SCIENCE OF SKINCARE LLC (006251958)