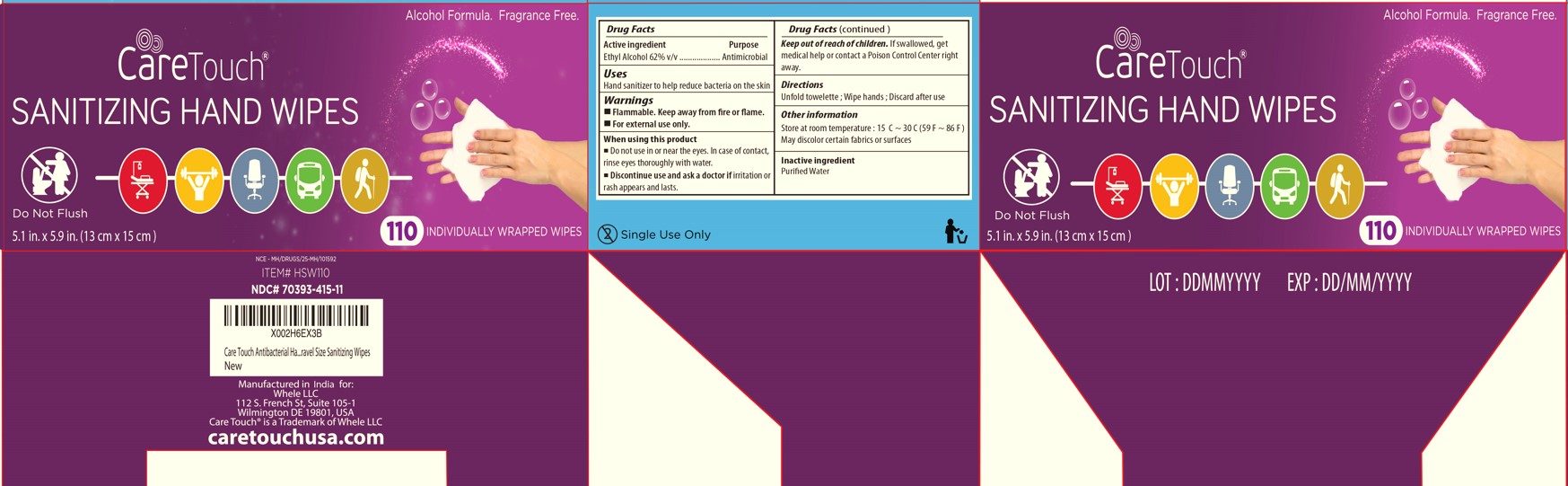
Label: CARETOUCH- ethyl alcohol cloth
-
NDC Code(s):
70393-415-01,
70393-415-11,
70393-415-21,
70393-415-22, view more70393-415-30
- Packager: Future Diagnostics Llc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredient
- Other information
-
SPL UNCLASSIFIED SECTION
Alcohol Formula. Fragrance Free. Paraben Free
INDIVIDUALLY WRAPPED WIPES
KILLS 99.99% OF GERMS
Take Your Care On the Go
Care Touch Sanitizing Hand Wipes kill 99.99% germs, remove dirt, clean messes, and sanitize all in one quick wipe. They're the perfect on-the go wipes to bring anywhere and everywhere. Whether you're at home, work, the gym, on the train, or even at the airport (these wipes breeze through airport security), feel clean within seconds by taking these wipes everywhere you go.
Each unscented wipe is large and moisturized enough to clean your hands and more; so even one goes a long way! Unlike other wipes, our formula won't leave your hands feeling rough, and smelling even worse.
Each wipe comes with our pledge to maintain the highest standards of quality, and is the results of extensive research, experience, dedication, and love.
1800.758.3830
Manufactured in China for Future Diagnostics LLC Brooklyn, NY 11220
futurediagnosticsusa.com
- Packaging
-
INGREDIENTS AND APPEARANCE
CARETOUCH
ethyl alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70393-415 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70393-415-11 110 in 1 BOX 05/23/2017 1 NDC:70393-415-01 1 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:70393-415-21 210 in 1 BOX 05/23/2017 2 NDC:70393-415-01 1 mL in 1 PACKET; Type 0: Not a Combination Product 3 NDC:70393-415-30 30 in 1 BOX 05/23/2017 3 NDC:70393-415-01 1 mL in 1 PACKET; Type 0: Not a Combination Product 4 NDC:70393-415-22 220 in 1 BOX 05/23/2017 4 NDC:70393-415-01 1 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/23/2017 Labeler - Future Diagnostics Llc (080113296)