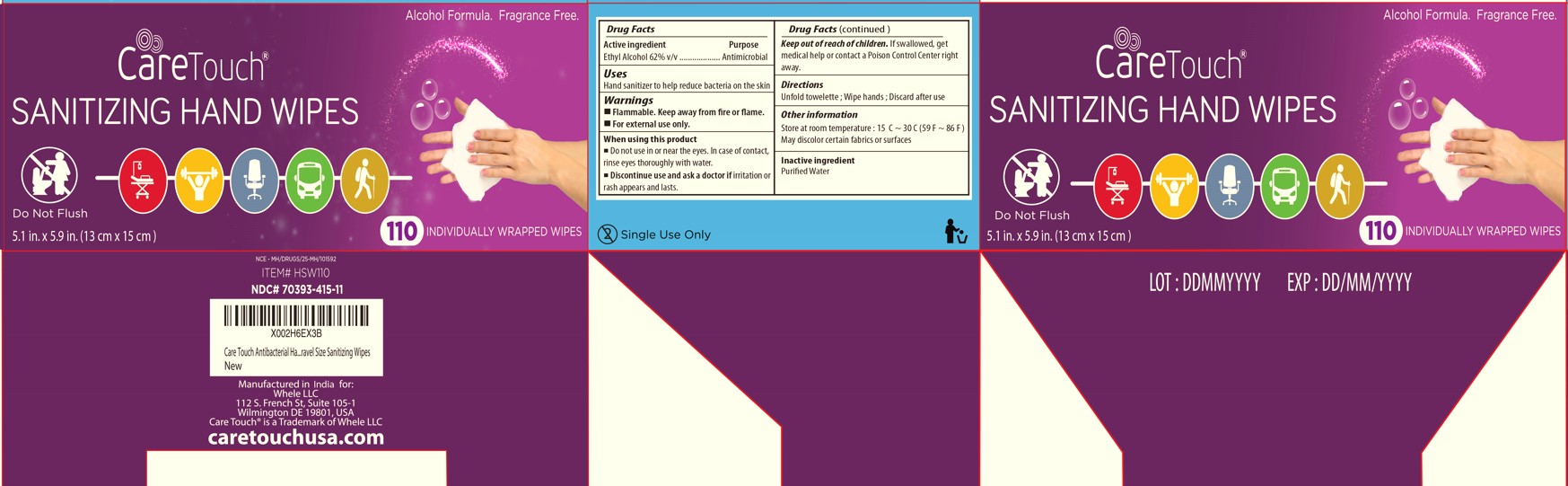
Warnings
■
Flammable. Keep away from fire or flame.
■ For external use only.
When using this product
■ Do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
■
Discontinue use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Other information
■ Store at room temperature: 15℃~30℃(59℉~86℉)
■ May discolor certain fabrics or surfaces
Alcohol Formula. Fragrance Free. Paraben Free
INDIVIDUALLY WRAPPED WIPES
KILLS 99.99% OF GERMS
Take Your Care On the Go
Care Touch Sanitizing Hand Wipes kill 99.99% germs, remove dirt, clean messes, and sanitize all in one quick wipe. They're the perfect on-the go wipes to bring anywhere and everywhere. Whether you're at home, work, the gym, on the train, or even at the airport (these wipes breeze through airport security), feel clean within seconds by taking these wipes everywhere you go.
Each unscented wipe is large and moisturized enough to clean your hands and more; so even one goes a long way! Unlike other wipes, our formula won't leave your hands feeling rough, and smelling even worse.
Each wipe comes with our pledge to maintain the highest standards of quality, and is the results of extensive research, experience, dedication, and love.
1800.758.3830
Manufactured in China for Future Diagnostics LLC Brooklyn, NY 11220
futurediagnosticsusa.com