Label: ACUTE-KARE HEALTHCARE PERSONNEL HANDWASH- chloroxylenol soap
- NDC Code(s): 11084-800-13, 11084-800-41
- Packager: SC Johnson Professional USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product do not get it in the eyes; this product causes eye irritation upon direct contact. In case of eye exposure, rinse thoroughly with water. If eye irritation persists, contact a physician.
- Directions
- Inactive ingredients
- Questions or comments?
-
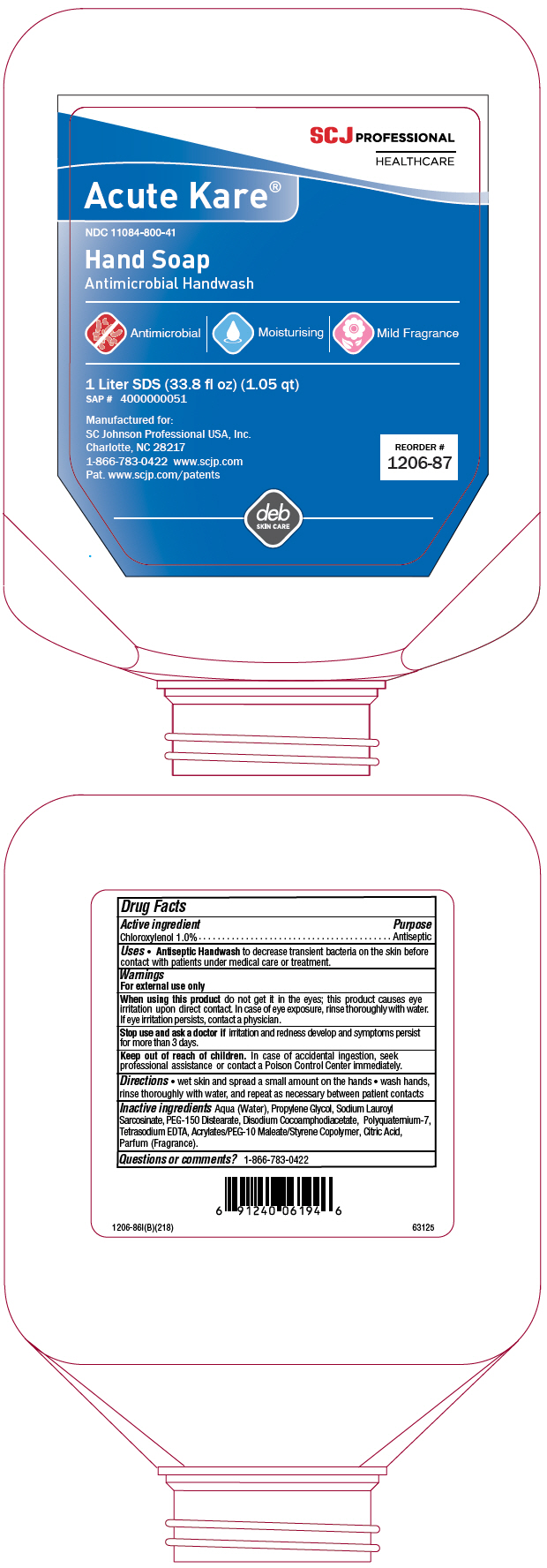
PRINCIPAL DISPLAY PANEL - 1 Liter Bottle Label
SCJ PROFESSIONAL
HEALTHCAREAcute Kare®
NDC 11084-800-41
Hand Soap
Antimicrobial HandwashAntimicrobial | Moisturising | Mild Fragrance
1 Liter SDS (33.8 fl oz) (1.05 qt)
SAP # 4000000051Manufactured for:
SC Johnson Professional USA, Inc.
Charlotte, NC 28217
1-866-783-0422 www.scjp.com
Pat. www.scjp.com/patentsREORDER #
1206-87deb
SKIN CARE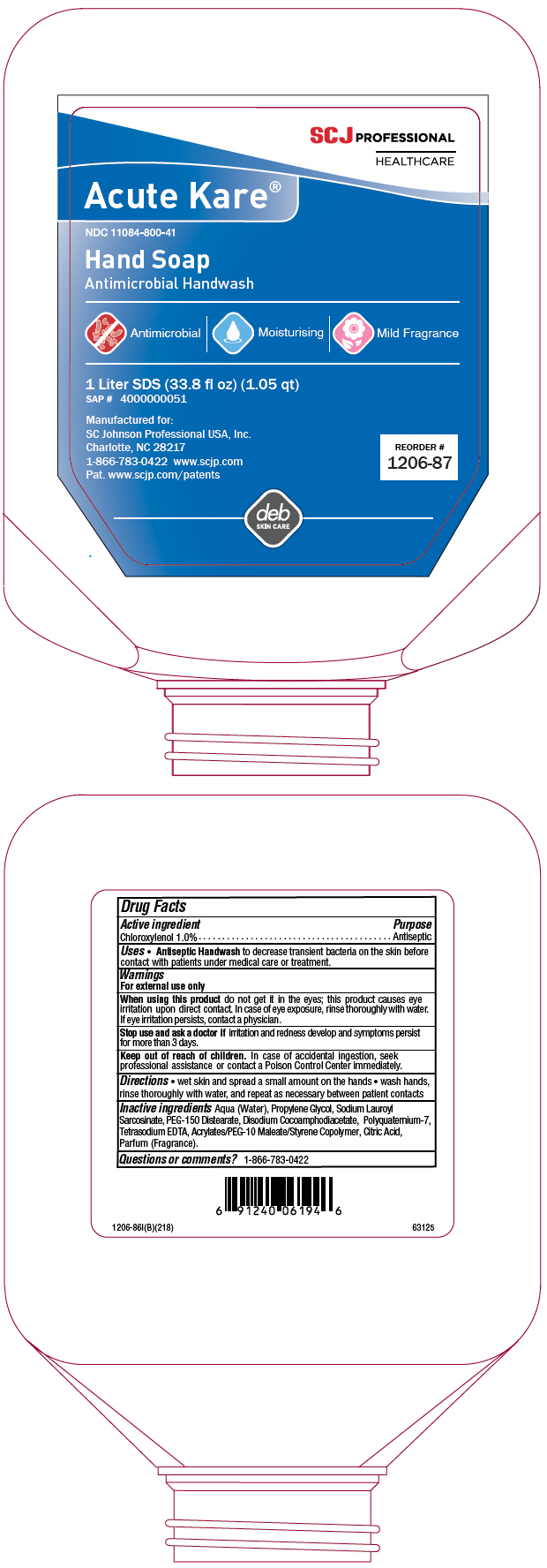
-
INGREDIENTS AND APPEARANCE
ACUTE-KARE HEALTHCARE PERSONNEL HANDWASH
chloroxylenol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11084-800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) EDETATE SODIUM (UNII: MP1J8420LU) METHACRYLATE/METHOXY PEG-10 MALEATE/STYRENE COPOLYMER (UNII: 39DK5WQ2PR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11084-800-13 444 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2018 01/01/2022 2 NDC:11084-800-41 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 02/01/2018 Labeler - SC Johnson Professional USA, Inc. (607378015) Establishment Name Address ID/FEI Business Operations STERIS Corporation 139424188 MANUFACTURE(11084-800) Establishment Name Address ID/FEI Business Operations APEX International, Inc. 015226132 MANUFACTURE(11084-800)