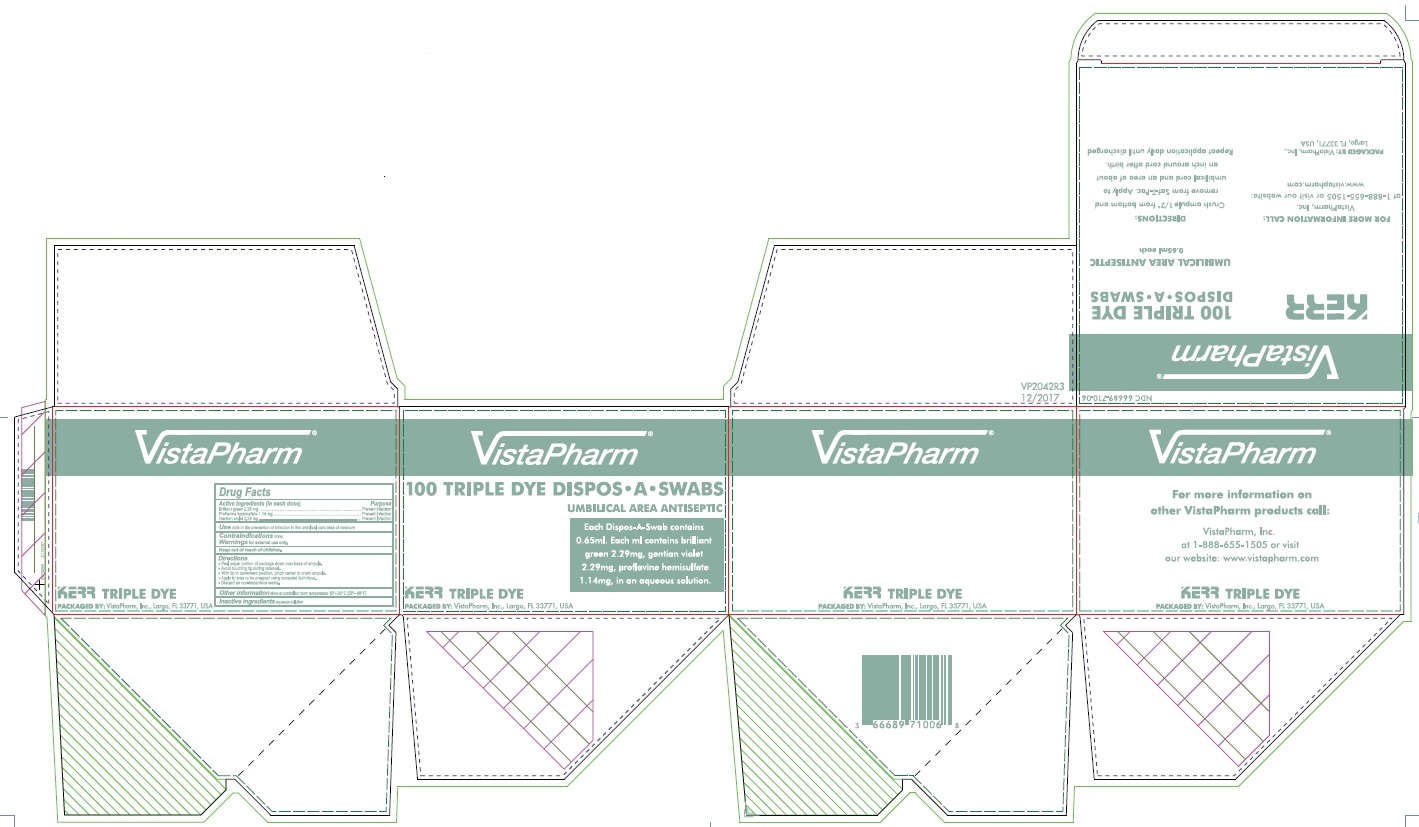
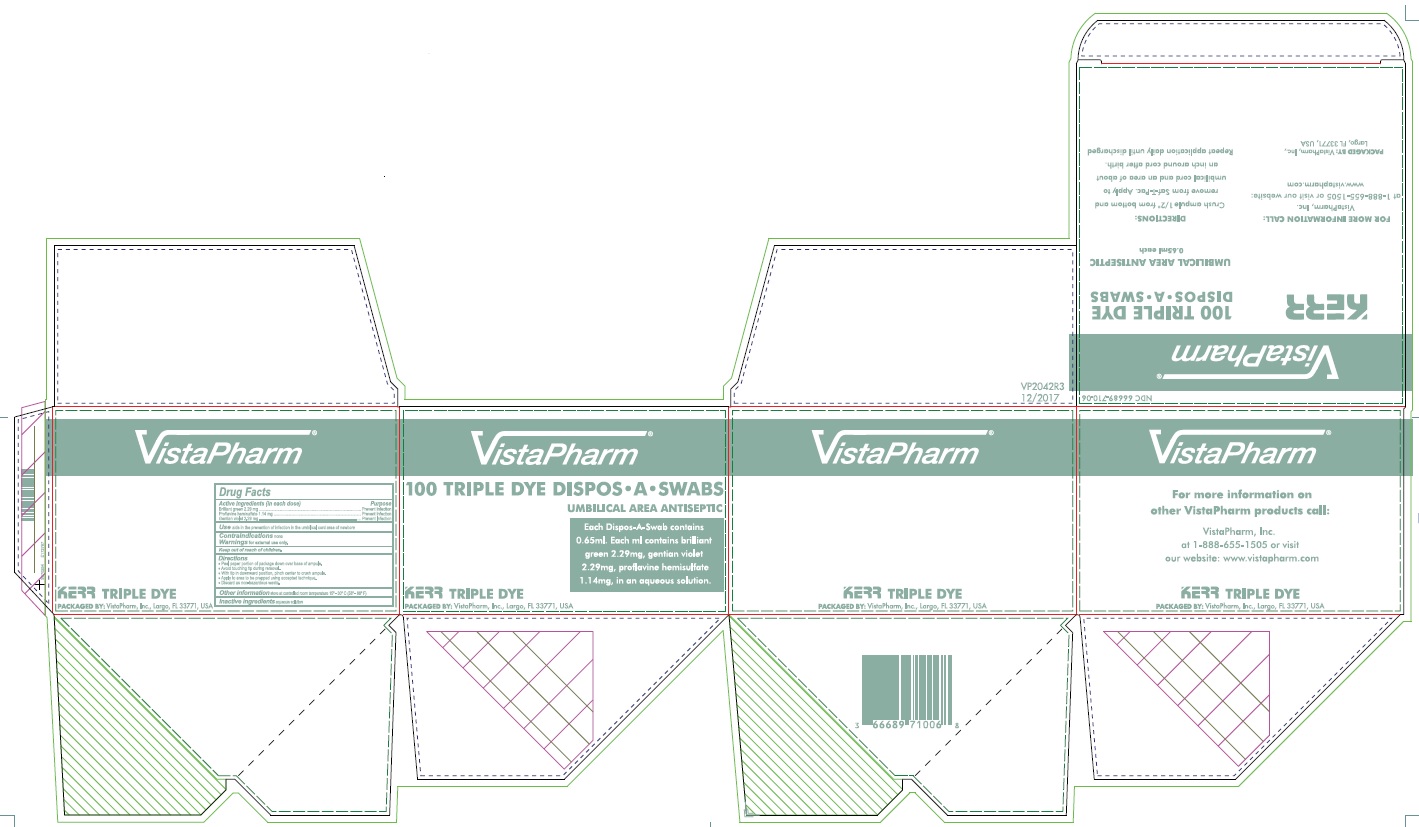
Label: KERR 100 TRIPLE DYE DISPOS-A- proflavine hemisulfate, brilliant green, and gentian violet swab
- NDC Code(s): 66689-710-06
- Packager: VistaPharm, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Contraindications
- Warnings
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 100 Swab Box
-
INGREDIENTS AND APPEARANCE
KERR 100 TRIPLE DYE DISPOS-A
proflavine hemisulfate, brilliant green, and gentian violet swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66689-710 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROFLAVINE HEMISULFATE (UNII: 27V8M747VB) (PROFLAVINE - UNII:CY3RNB3K4T) PROFLAVINE HEMISULFATE 1.14 mg in 1 mL BRILLIANT GREEN (UNII: G0L543D370) (BRILLIANT GREEN CATION - UNII:S85JD8CLBH) BRILLIANT GREEN 2.29 mg in 1 mL GENTIAN VIOLET (UNII: J4Z741D6O5) (GENTIAN VIOLET CATION - UNII:3GVJ31T6YY) GENTIAN VIOLET 2.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color green (Violet) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66689-710-06 100 in 1 BOX 05/01/2004 1 1 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2004 Labeler - VistaPharm, LLC (116743084)