Directions
- Peel paper portion of package down over base of ampule.
- Avoid touching tip during removal.
- With tip in downward position, pinch center to crush ampule.
- Apply to area to be prepped using accepted technique.
- Discard as non-hazardous waste.
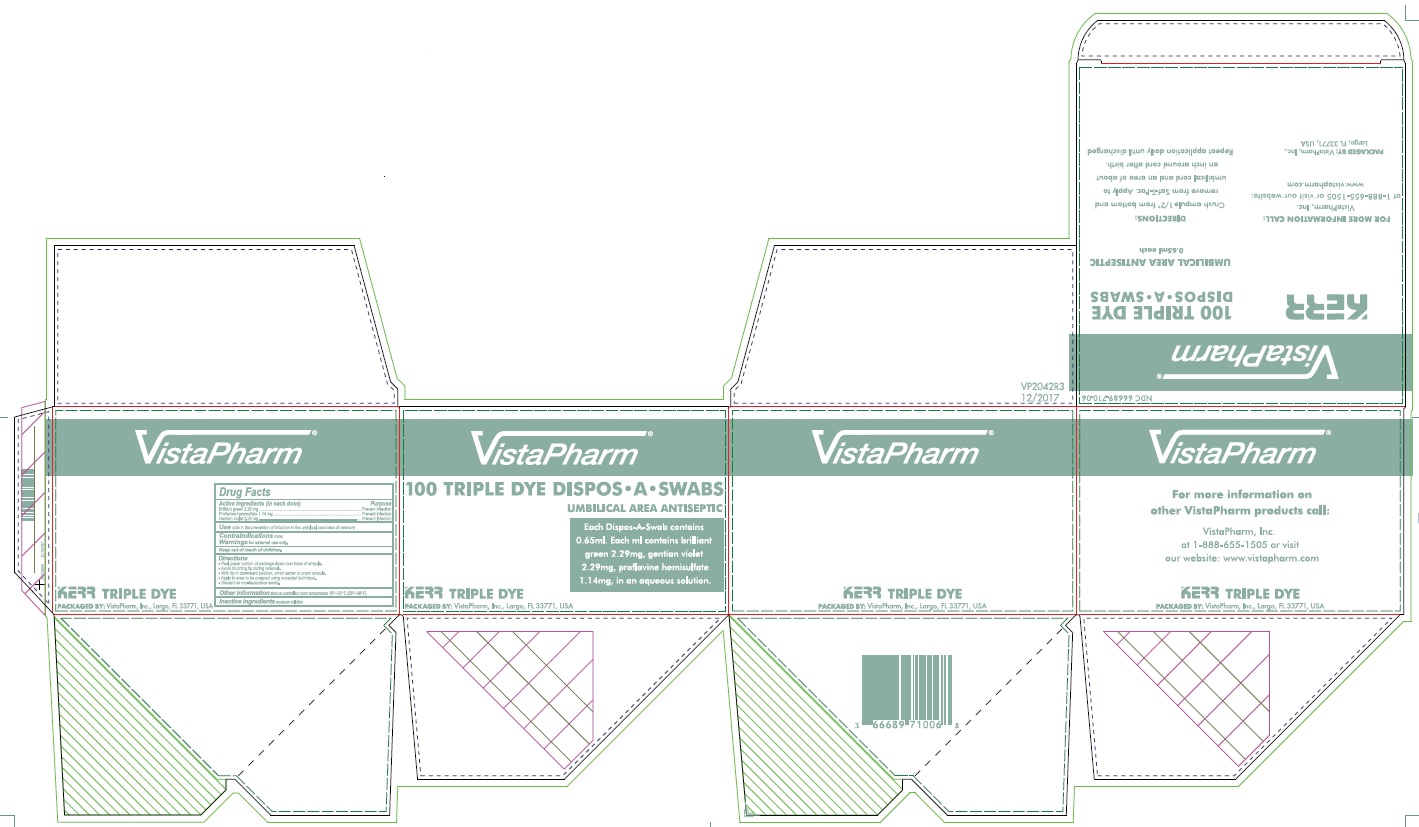
PRINCIPAL DISPLAY PANEL - 100 Swab Box
VistaPharm®
100 TRIPLE DYE DISPOS•A•SWABS
UMBILICAL AREA ANTISEPTIC
Each Dispos-A-Swab contains
0.65ml. Each ml contains brilliant
green 2.29mg, gentian violet
2.29mg, proflavine hemisulfate
1.14mg, in an aqueous solution.
KERR TRIPLE DYE
PACKAGED BY: VistaPharm, Inc., Largo, FL 33771, USA